+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: SASBDB / ID: SASDAW6 |
|---|---|
 試料 試料 | Iron-sulfur cluster assembly scaffold IscU monomer
|
| 機能・相同性 |  機能・相同性情報 機能・相同性情報IscS-IscU complex / L-cysteine catabolic process / iron-sulfur cluster assembly / ferrous iron binding / 2 iron, 2 sulfur cluster binding / 4 iron, 4 sulfur cluster binding / intracellular iron ion homeostasis / copper ion binding / zinc ion binding / identical protein binding ...IscS-IscU complex / L-cysteine catabolic process / iron-sulfur cluster assembly / ferrous iron binding / 2 iron, 2 sulfur cluster binding / 4 iron, 4 sulfur cluster binding / intracellular iron ion homeostasis / copper ion binding / zinc ion binding / identical protein binding / cytosol / cytoplasm 類似検索 - 分子機能 |
| 生物種 |  |
 引用 引用 |  ジャーナル: Nat Commun / 年: 2010 ジャーナル: Nat Commun / 年: 2010タイトル: Structural bases for the interaction of frataxin with the central components of iron-sulphur cluster assembly. 著者: Filippo Prischi / Petr V Konarev / Clara Iannuzzi / Chiara Pastore / Salvatore Adinolfi / Stephen R Martin / Dmitri I Svergun / Annalisa Pastore /  要旨: Reduced levels of frataxin, an essential protein of as yet unknown function, are responsible for causing the neurodegenerative pathology Friedreich's ataxia. Independent reports have linked frataxin ...Reduced levels of frataxin, an essential protein of as yet unknown function, are responsible for causing the neurodegenerative pathology Friedreich's ataxia. Independent reports have linked frataxin to iron-sulphur cluster assembly through interactions with the two central components of this machinery: desulphurase Nfs1/IscS and the scaffold protein Isu/IscU. In this study, we use a combination of biophysical methods to define the structural bases of the interaction of CyaY (the bacterial orthologue of frataxin) with the IscS/IscU complex. We show that CyaY binds IscS as a monomer in a pocket between the active site and the IscS dimer interface. Recognition does not require iron and occurs through electrostatic interactions of complementary charged residues. Mutations at the complex interface affect the rates of enzymatic cluster formation. CyaY binding strengthens the affinity of the IscS/IscU complex. Our data suggest a new paradigm for understanding the role of frataxin as a regulator of IscS functions. |
 登録者 登録者 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-モデル
| モデル #223 | 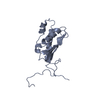 タイプ: atomic / ソフトウェア: crysol / ダミー原子の半径: 1.90 A / 対称性: P1 / カイ2乗値: 2.199289  Omokage検索でこの集合体の類似形状データを探す (詳細) Omokage検索でこの集合体の類似形状データを探す (詳細) |
|---|---|
| モデル #224 |  タイプ: dummy / ソフトウェア: DAMMIF / ダミー原子の半径: 2.30 A / 対称性: P1 / カイ2乗値: 1.065024  Omokage検索でこの集合体の類似形状データを探す (詳細) Omokage検索でこの集合体の類似形状データを探す (詳細) |
- 試料
試料
 試料 試料 | 名称: Iron-sulfur cluster assembly scaffold IscU monomer / 試料濃度: 2.5 mg/ml |
|---|---|
| バッファ | 名称: Tris-HCl / 濃度: 20.00 mM / pH: 8 / 組成: 150 mM NaCl and 10 mM β-mercaptoethanol |
| 要素 #136 | タイプ: protein / 記述: Iron-sulfur cluster assembly scaffold protein IscU / 分子量: 14.464 / 分子数: 1 / 由来: Escherichia coli / 参照: UniProt: P0ACD4 配列: MAYSEKVIDH YENPRNVGSL DKKDSNVGTG MVGAPACGDV MQLQIKVDDN GIIEDAKFKT YGCGSAIASS SLITEWVKGK SLEEAGAIKN SQIAEELELP PVKVHCSILA EDAIKAAIAD YKAKQGLEHH HHHH |
-実験情報
| ビーム | 設備名称:  DORIS III X33 DORIS III X33  / 地域: Hamburg / 国: Germany / 地域: Hamburg / 国: Germany  / 形状: 0.6 / 線源: X-ray synchrotron / 波長: 0.15 Å / スペクトロメータ・検出器間距離: 2.7 mm / 形状: 0.6 / 線源: X-ray synchrotron / 波長: 0.15 Å / スペクトロメータ・検出器間距離: 2.7 mm | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 検出器 | 名称: Pilatus 1M-W / Pixsize x: 0.172 mm | |||||||||||||||||||||||||||
| スキャン |
| |||||||||||||||||||||||||||
| 距離分布関数 P(R) |
| |||||||||||||||||||||||||||
| 結果 | コメント: Reduced levels of frataxin, an essential protein of as yet unknown function, are responsible for causing the neurodegenerative pathology Friedreich’s ataxia. Independent reports have ...コメント: Reduced levels of frataxin, an essential protein of as yet unknown function, are responsible for causing the neurodegenerative pathology Friedreich’s ataxia. Independent reports have linked frataxin to iron–sulphur cluster assembly through interactions with the two central components of this machinery: desulphurase Nfs1/IscS and the scaffold protein Isu/IscU. In this study, we use a combination of biophysical methods to define the structural bases of the interaction of CyaY (the bacterial orthologue of frataxin) with the IscS/IscU complex. We show that CyaY binds IscS as a monomer in a pocket between the active site and the IscS dimer interface. Recognition does not require iron and occurs through electrostatic interactions of complementary charged residues. Mutations at the complex interface affect the rates of enzymatic cluster formation. CyaY binding strengthens the affinity of the IscS/IscU complex. Our data suggest a new paradigm for understanding the role of frataxin as a regulator of IscS functions.
|
 ムービー
ムービー コントローラー
コントローラー


 SASDAW6
SASDAW6














