+ Open data
Open data
- Basic information
Basic information
| Entry | Database: SASBDB / ID: SASDAW6 |
|---|---|
 Sample Sample | Iron-sulfur cluster assembly scaffold IscU monomer
|
| Function / homology |  Function and homology information Function and homology informationIscS-IscU complex / L-cysteine catabolic process / iron-sulfur cluster assembly / ferrous iron binding / 2 iron, 2 sulfur cluster binding / 4 iron, 4 sulfur cluster binding / intracellular iron ion homeostasis / copper ion binding / zinc ion binding / identical protein binding ...IscS-IscU complex / L-cysteine catabolic process / iron-sulfur cluster assembly / ferrous iron binding / 2 iron, 2 sulfur cluster binding / 4 iron, 4 sulfur cluster binding / intracellular iron ion homeostasis / copper ion binding / zinc ion binding / identical protein binding / cytoplasm / cytosol Similarity search - Function |
| Biological species |  |
 Citation Citation |  Journal: Nat Commun / Year: 2010 Journal: Nat Commun / Year: 2010Title: Structural bases for the interaction of frataxin with the central components of iron-sulphur cluster assembly. Authors: Filippo Prischi / Petr V Konarev / Clara Iannuzzi / Chiara Pastore / Salvatore Adinolfi / Stephen R Martin / Dmitri I Svergun / Annalisa Pastore /  Abstract: Reduced levels of frataxin, an essential protein of as yet unknown function, are responsible for causing the neurodegenerative pathology Friedreich's ataxia. Independent reports have linked frataxin ...Reduced levels of frataxin, an essential protein of as yet unknown function, are responsible for causing the neurodegenerative pathology Friedreich's ataxia. Independent reports have linked frataxin to iron-sulphur cluster assembly through interactions with the two central components of this machinery: desulphurase Nfs1/IscS and the scaffold protein Isu/IscU. In this study, we use a combination of biophysical methods to define the structural bases of the interaction of CyaY (the bacterial orthologue of frataxin) with the IscS/IscU complex. We show that CyaY binds IscS as a monomer in a pocket between the active site and the IscS dimer interface. Recognition does not require iron and occurs through electrostatic interactions of complementary charged residues. Mutations at the complex interface affect the rates of enzymatic cluster formation. CyaY binding strengthens the affinity of the IscS/IscU complex. Our data suggest a new paradigm for understanding the role of frataxin as a regulator of IscS functions. |
 Contact author Contact author |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
-Models
| Model #223 | 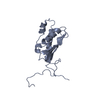 Type: atomic / Software: crysol / Radius of dummy atoms: 1.90 A / Symmetry: P1 / Chi-square value: 2.199289  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
|---|---|
| Model #224 |  Type: dummy / Software: DAMMIF / Radius of dummy atoms: 2.30 A / Symmetry: P1 / Chi-square value: 1.065024  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
- Sample
Sample
 Sample Sample | Name: Iron-sulfur cluster assembly scaffold IscU monomer / Specimen concentration: 2.5 mg/ml |
|---|---|
| Buffer | Name: Tris-HCl / Concentration: 20.00 mM / pH: 8 / Composition: 150 mM NaCl and 10 mM β-mercaptoethanol |
| Entity #136 | Type: protein Description: Iron-sulfur cluster assembly scaffold protein IscU Formula weight: 14.464 / Num. of mol.: 1 / Source: Escherichia coli / References: UniProt: P0ACD4 Sequence: MAYSEKVIDH YENPRNVGSL DKKDSNVGTG MVGAPACGDV MQLQIKVDDN GIIEDAKFKT YGCGSAIASS SLITEWVKGK SLEEAGAIKN SQIAEELELP PVKVHCSILA EDAIKAAIAD YKAKQGLEHH HHHH |
-Experimental information
| Beam | Instrument name:  DORIS III X33 DORIS III X33  / City: Hamburg / 国: Germany / City: Hamburg / 国: Germany  / Shape: 0.6 / Type of source: X-ray synchrotron / Wavelength: 0.15 Å / Dist. spec. to detc.: 2.7 mm / Shape: 0.6 / Type of source: X-ray synchrotron / Wavelength: 0.15 Å / Dist. spec. to detc.: 2.7 mm | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detector | Name: Pilatus 1M-W / Pixsize x: 0.172 mm | |||||||||||||||||||||||||||
| Scan |
| |||||||||||||||||||||||||||
| Distance distribution function P(R) |
| |||||||||||||||||||||||||||
| Result | Comments: Reduced levels of frataxin, an essential protein of as yet unknown function, are responsible for causing the neurodegenerative pathology Friedreich’s ataxia. Independent reports have ...Comments: Reduced levels of frataxin, an essential protein of as yet unknown function, are responsible for causing the neurodegenerative pathology Friedreich’s ataxia. Independent reports have linked frataxin to iron–sulphur cluster assembly through interactions with the two central components of this machinery: desulphurase Nfs1/IscS and the scaffold protein Isu/IscU. In this study, we use a combination of biophysical methods to define the structural bases of the interaction of CyaY (the bacterial orthologue of frataxin) with the IscS/IscU complex. We show that CyaY binds IscS as a monomer in a pocket between the active site and the IscS dimer interface. Recognition does not require iron and occurs through electrostatic interactions of complementary charged residues. Mutations at the complex interface affect the rates of enzymatic cluster formation. CyaY binding strengthens the affinity of the IscS/IscU complex. Our data suggest a new paradigm for understanding the role of frataxin as a regulator of IscS functions.
|
 Movie
Movie Controller
Controller


 SASDAW6
SASDAW6














