[English] 日本語
 Yorodumi
Yorodumi- PDB-9g0b: Rhinovirus A2 uncoating intermediate revealing the natural pocket... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9g0b | ||||||
|---|---|---|---|---|---|---|---|
| Title | Rhinovirus A2 uncoating intermediate revealing the natural pocket factor (pH 5.8 and 4 degrees Celsius) | ||||||
 Components Components |
| ||||||
 Keywords Keywords | VIRUS / Uncoating Intermediate / Pocket Factor / Lauric Acid | ||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / ribonucleoside triphosphate phosphatase activity / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / channel activity ...symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / ribonucleoside triphosphate phosphatase activity / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / DNA replication / RNA helicase activity / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | ||||||
| Biological species |  rhinovirus A2 rhinovirus A2 | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.2 Å | ||||||
 Authors Authors | Real-Hohn, A. / Blaas, D. | ||||||
| Funding support |  Austria, 1items Austria, 1items
| ||||||
 Citation Citation |  Journal: Sci Rep / Year: 2025 Journal: Sci Rep / Year: 2025Title: New rhinovirus uncoating intermediate reveals how sodium versus potassium ions influence RNA release. Authors: Antonio Real-Hohn / Dieter Blaas /  Abstract: Electron microscopy (EM) of rhinovirus A2 (RV-A2) incubated in Na phosphate buffer (pH 7.6) for 12 h at 25 °C revealed partial fragmentation, whereas upon incubation in K phosphate buffer, RV-A2 ...Electron microscopy (EM) of rhinovirus A2 (RV-A2) incubated in Na phosphate buffer (pH 7.6) for 12 h at 25 °C revealed partial fragmentation, whereas upon incubation in K phosphate buffer, RV-A2 appeared intact. In buffers adjusted to pH 5.8, these differences became more pronounced; acidic Na phosphate buffer promoted disintegration of the particles, whereas in acidic K phosphate buffer, the virus appeared like native. Incubation in the acidic buffers for one hour at 4 °C followed by neutralisation resulted in the respective formation of non-infectious A particles (in Na) and a non-infectious novel uncoating intermediate (in K), which we termed 'E0 particle'. Negative staining EM revealed phosphotungstate penetration into A particles, but not into E0 particles. Cryo-EM image reconstruction of the E0 particle showed clear differences between A and E0 particles; like native virus, E0 contained VP4 and a pocket factor. Native RV-A2 RNA cores, obtained by gentle proteinase-K digestion in K and Na phosphate buffer, respectively, differed in accessibility of dsRNA regions, detected by PaSTRy. Variance in RNA compactness observed in K versus Na phosphate buffer was confirmed by rotary shadowing EM; in K phosphate buffer, the RNA remained condensed while, in Na phosphate buffer, distinct unfolding stages were apparent. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9g0b.cif.gz 9g0b.cif.gz | 173.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9g0b.ent.gz pdb9g0b.ent.gz | 134.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9g0b.json.gz 9g0b.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/g0/9g0b https://data.pdbj.org/pub/pdb/validation_reports/g0/9g0b ftp://data.pdbj.org/pub/pdb/validation_reports/g0/9g0b ftp://data.pdbj.org/pub/pdb/validation_reports/g0/9g0b | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  50930MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 | x 60
|
- Components
Components
| #1: Protein | Mass: 32274.100 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  rhinovirus A2 / References: UniProt: P04936 rhinovirus A2 / References: UniProt: P04936 |
|---|---|
| #2: Protein | Mass: 29009.588 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  rhinovirus A2 / References: UniProt: P04936 rhinovirus A2 / References: UniProt: P04936 |
| #3: Protein | Mass: 26107.793 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  rhinovirus A2 / References: UniProt: P04936 rhinovirus A2 / References: UniProt: P04936 |
| #4: Protein | Mass: 7356.971 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  rhinovirus A2 / References: UniProt: P04936 rhinovirus A2 / References: UniProt: P04936 |
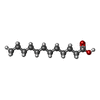
| #5: Chemical | ChemComp-DAO / |
| Has ligand of interest | Y |
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: rhinovirus A2 / Type: VIRUS / Entity ID: #1-#4 / Source: NATURAL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | |||||||||||||||
| Source (natural) | Organism:  rhinovirus A2 rhinovirus A2 | |||||||||||||||
| Details of virus | Empty: NO / Enveloped: NO / Isolate: SEROTYPE / Type: VIRION | |||||||||||||||
| Natural host | Organism: Homo sapiens | |||||||||||||||
| Virus shell | Diameter: 310 nm / Triangulation number (T number): 3 | |||||||||||||||
| Buffer solution | pH: 5.8 / Details: 0.1 M phosphate buffer, pH 5.8 | |||||||||||||||
| Buffer component |
| |||||||||||||||
| Specimen | Conc.: 10 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: The virus sample was purified from HeLa cells, diluted to a final concentration of 1 mg/mL in 100 mM potassium phosphate buffer, pH 5.8, and incubated at four degrees for one hour before the vitrification. | |||||||||||||||
| Specimen support | Grid material: COPPER / Grid mesh size: 200 divisions/in. / Grid type: Quantifoil R2/2 | |||||||||||||||
| Vitrification | Instrument: LEICA EM GP / Cryogen name: ETHANE / Humidity: 80 % / Chamber temperature: 293 K |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: TFS GLACIOS |
|---|---|
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 100000 X / Nominal defocus max: 2500 nm / Nominal defocus min: 500 nm / Cs: 2.7 mm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN |
| Image recording | Electron dose: 60 e/Å2 / Detector mode: INTEGRATING / Film or detector model: FEI FALCON III (4k x 4k) / Num. of grids imaged: 2 / Num. of real images: 246 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 16372 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 9825 / Algorithm: FOURIER SPACE / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT / Space: REAL | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 3VDD Accession code: 3VDD / Source name: PDB / Type: experimental model |
 Movie
Movie Controller
Controller


 PDBj
PDBj