[English] 日本語
 Yorodumi
Yorodumi- PDB-8rvy: CryoEM structure of the Elp-Hdr complex of Methanothermobacter ma... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8rvy | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of the Elp-Hdr complex of Methanothermobacter marburgensis state 1 (composite structure) | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords | OXIDOREDUCTASE / Redox / Flavin-based electron bifurcation / methanogenesis / heterodisulfide reductase / F420-H2 oxidase | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationformate dehydrogenase (coenzyme F420) / formate dehydrogenase (coenzyme F420) activity / H2:CoB-CoM heterodisulfide,ferredoxin reductase / CoB--CoM heterodisulfide reductase activity / oxidoreductase activity, acting on CH or CH2 groups, with an iron-sulfur protein as acceptor / formate dehydrogenase / methanogenesis / NADH dehydrogenase activity / iron-sulfur cluster binding / respiratory electron transport chain ...formate dehydrogenase (coenzyme F420) / formate dehydrogenase (coenzyme F420) activity / H2:CoB-CoM heterodisulfide,ferredoxin reductase / CoB--CoM heterodisulfide reductase activity / oxidoreductase activity, acting on CH or CH2 groups, with an iron-sulfur protein as acceptor / formate dehydrogenase / methanogenesis / NADH dehydrogenase activity / iron-sulfur cluster binding / respiratory electron transport chain / 2 iron, 2 sulfur cluster binding / 4 iron, 4 sulfur cluster binding / oxidoreductase activity / metal ion binding / membrane / plasma membrane Similarity search - Function | |||||||||||||||
| Biological species |   Methanothermobacter marburgensis (archaea) Methanothermobacter marburgensis (archaea) | |||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.36 Å | |||||||||||||||
 Authors Authors | San Segundo-Acosta, P. / Murphy, B.J. | |||||||||||||||
| Funding support |  Germany, 1items Germany, 1items
| |||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2025 Journal: Nature / Year: 2025Title: Electron flow in hydrogenotrophic methanogens under nickel limitation. Authors: Shunsuke Nomura / Pablo San Segundo-Acosta / Evgenii Protasov / Masanori Kaneko / Jörg Kahnt / Bonnie J Murphy / Seigo Shima /    Abstract: Methanogenic archaea are the main producers of the potent greenhouse gas methane. In the methanogenic pathway from CO and H studied under laboratory conditions, low-potential electrons for CO ...Methanogenic archaea are the main producers of the potent greenhouse gas methane. In the methanogenic pathway from CO and H studied under laboratory conditions, low-potential electrons for CO reduction are generated by a flavin-based electron-bifurcation reaction catalysed by heterodisulfide reductase (Hdr) complexed with the associated [NiFe]-hydrogenase (Mvh). F-reducing [NiFe]-hydrogenase (Frh) provides electrons to the methanogenic pathway through the electron carrier F (ref. ). Here we report that under strictly nickel-limited conditions, in which the nickel concentration is similar to those often observed in natural habitats, the production of both [NiFe]-hydrogenases in Methanothermobacter marburgensis is strongly downregulated. The Frh reaction is substituted by a coupled reaction with [Fe]-hydrogenase (Hmd), and the role of Mvh is taken over by F-dependent electron-donating proteins (Elp). Thus, Hmd provides all electrons for the reducing metabolism under these nickel-limited conditions. Biochemical and structural characterization of Elp-Hdr complexes confirms the electronic interaction between Elp and Hdr. The conservation of the genes encoding Elp and Hmd in CO-reducing hydrogenotrophic methanogens suggests that the Hmd system is an alternative pathway for electron flow in CO-reducing hydrogenotrophic methanogens under nickel-limited conditions. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8rvy.cif.gz 8rvy.cif.gz | 1 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8rvy.ent.gz pdb8rvy.ent.gz | 868.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8rvy.json.gz 8rvy.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rv/8rvy https://data.pdbj.org/pub/pdb/validation_reports/rv/8rvy ftp://data.pdbj.org/pub/pdb/validation_reports/rv/8rvy ftp://data.pdbj.org/pub/pdb/validation_reports/rv/8rvy | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  19538MC  8rvuC  8rvvC  8rwnC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-H(2):CoB-CoM heterodisulfide,ferredoxin reductase subunit ... , 3 types, 6 molecules AabBcC
| #1: Protein | Mass: 72264.961 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)   Methanothermobacter marburgensis (archaea) Methanothermobacter marburgensis (archaea)References: UniProt: Q50756, H2:CoB-CoM heterodisulfide,ferredoxin reductase #5: Protein | Mass: 33496.371 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)   Methanothermobacter marburgensis (archaea) Methanothermobacter marburgensis (archaea)References: UniProt: Q50755, H2:CoB-CoM heterodisulfide,ferredoxin reductase #6: Protein | Mass: 20548.727 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)   Methanothermobacter marburgensis (archaea) Methanothermobacter marburgensis (archaea)References: UniProt: Q50754, H2:CoB-CoM heterodisulfide,ferredoxin reductase |
|---|
-Formate dehydrogenase, ... , 2 types, 2 molecules ED
| #2: Protein | Mass: 43387.148 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)   Methanothermobacter marburgensis (archaea) Methanothermobacter marburgensis (archaea)References: UniProt: D9PY03, formate dehydrogenase |
|---|---|
| #3: Protein | Mass: 37682.695 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)   Methanothermobacter marburgensis (archaea) Methanothermobacter marburgensis (archaea)References: UniProt: D9PY04, formate dehydrogenase |
-Protein , 1 types, 1 molecules F
| #4: Protein | Mass: 15550.847 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)   Methanothermobacter marburgensis (archaea) Methanothermobacter marburgensis (archaea)References: UniProt: D9PY02 |
|---|
-Non-polymers , 5 types, 40 molecules 


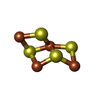





| #7: Chemical | ChemComp-SF4 / #8: Chemical | #9: Chemical | ChemComp-FES / | #10: Chemical | ChemComp-9S8 / #11: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | N |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: F420-dependent electron-donating proteins- heterodisulfide reductase complex (Elp-Hdr) from Methanothermobacter marburgensis (heterodisulfide reductase core and mobile arm, composite structure) Type: COMPLEX / Entity ID: #1-#6 / Source: NATURAL |
|---|---|
| Molecular weight | Value: 0.45 MDa / Experimental value: NO |
| Source (natural) | Organism:   Methanothermobacter marburgensis (archaea) Methanothermobacter marburgensis (archaea) |
| Buffer solution | pH: 7.6 Details: 50 mM Tris-HCl pH 7.6 containing 400 mM Ammonium sulfate. |
| Specimen | Conc.: 1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: NITROGEN / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2200 nm / Nominal defocus min: 800 nm / Cs: 2.7 mm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 65 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 7768 |
| EM imaging optics | Energyfilter name: GIF Bioquantum |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.36 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 76528 / Symmetry type: POINT | ||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT / Space: REAL | ||||||||||||||||||||||||
| Atomic model building | Details: Automated model building using Model Angelo / Source name: Other / Type: other | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj
















