+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8pjk | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | ST171-bound serotonin 5-HT1A receptor - Gi Protein Complex | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of serotonin secretion / Gi/o-coupled serotonin receptor activity / regulation of hormone secretion / regulation of behavior / receptor-receptor interaction / Serotonin receptors / regulation of dopamine metabolic process / serotonin receptor activity / G protein-coupled serotonin receptor activity / serotonin receptor signaling pathway ...regulation of serotonin secretion / Gi/o-coupled serotonin receptor activity / regulation of hormone secretion / regulation of behavior / receptor-receptor interaction / Serotonin receptors / regulation of dopamine metabolic process / serotonin receptor activity / G protein-coupled serotonin receptor activity / serotonin receptor signaling pathway / neurotransmitter receptor activity / serotonin metabolic process / serotonin binding / gamma-aminobutyric acid signaling pathway / exploration behavior / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / regulation of vasoconstriction / behavioral fear response / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / G protein-coupled serotonin receptor binding / adenylate cyclase regulator activity / adenylate cyclase-inhibiting serotonin receptor signaling pathway / cellular response to forskolin / regulation of mitotic spindle organization / Regulation of insulin secretion / positive regulation of cholesterol biosynthetic process / negative regulation of insulin secretion / G protein-coupled receptor binding / response to peptide hormone / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / Activation of the phototransduction cascade / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G-protein activation / G beta:gamma signalling through CDC42 / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / G beta:gamma signalling through BTK / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Sensory perception of sweet, bitter, and umami (glutamate) taste / Glucagon-type ligand receptors / GDP binding / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADP signalling through P2Y purinoceptor 1 / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / Inactivation, recovery and regulation of the phototransduction cascade / G alpha (12/13) signalling events / extracellular vesicle / sensory perception of taste / Thrombin signalling through proteinase activated receptors (PARs) / signaling receptor complex adaptor activity / G protein activity / retina development in camera-type eye / GTPase binding / Ca2+ pathway / fibroblast proliferation / midbody / cell cortex / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / G alpha (q) signalling events / chemical synaptic transmission / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / Ras protein signal transduction / Extra-nuclear estrogen signaling / cell population proliferation / ciliary basal body / G protein-coupled receptor signaling pathway / lysosomal membrane / cell division / GTPase activity / positive regulation of cell population proliferation Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.4 Å | |||||||||
 Authors Authors | Schneider, J. / Gmeiner, P. / Rasmussen, T. / Boettcher, B. | |||||||||
| Funding support | European Union,  Germany, 2items Germany, 2items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2025 Journal: Sci Adv / Year: 2025Title: Discovery of a functionally selective serotonin receptor (5-HTR) agonist for the treatment of pain. Authors: Annika Ullrich / Johannes Schneider / João M Braz / Eduard Neu / Nico Staffen / Markus Stanek / Jana Bláhová / Tamsanqa Hove / Tamara Albert / Anni Allikalt / Stefan Löber / Karnika ...Authors: Annika Ullrich / Johannes Schneider / João M Braz / Eduard Neu / Nico Staffen / Markus Stanek / Jana Bláhová / Tamsanqa Hove / Tamara Albert / Anni Allikalt / Stefan Löber / Karnika Bhardwaj / Sian Rodriguez-Rosado / Elissa Fink / Tim Rasmussen / Harald Hübner / Asuka Inoue / Brian K Shoichet / Allan I Basbaum / Bettina Böttcher / Dorothee Weikert / Peter Gmeiner /    Abstract: The heterotrimeric G protein-coupled serotonin receptor 5-HT receptor (5-HTR) mediates antinociception and may serve as a valuable target for the treatment of pain. Starting from a chemical library, ...The heterotrimeric G protein-coupled serotonin receptor 5-HT receptor (5-HTR) mediates antinociception and may serve as a valuable target for the treatment of pain. Starting from a chemical library, we evolved ST171, a bitopic 5-HTR agonist that revealed highly potent and functionally selective G signaling without G activation and marginal β-arrestin recruitment. ST171 is effective in acute and chronic pain models. Cryo-electron microscopy structures of ST171 bound to 5-HTR in complex with the G protein compared to the canonical agonist befiradol bound to complexes of 5-HTR with G or G revealed that the ligands occupy different exo-sites. The individual binding poses are associated with ligand-specific receptor conformations that were further studied by molecular dynamics simulations, allowing us to better understand ligand bias, a phenomenon that may be crucial to the discovery of more effective and safe G protein-coupled receptor drugs. #1:  Journal: Biorxiv / Year: 2023 Journal: Biorxiv / Year: 2023Title: Discovery of a functionally selective serotonin 5-HT1A receptor agonist for the treatment of pain Authors: Ullrich, A. / Schneider, J. / Braz, J.M. / Neu, E. / Staffen, N. / Stanek, M. / Blahova, J. / Hove, T. / Albert, T. / Allikalt, A. / Lober, S. / Bhardwaj, K. / Rodriguez-Rosado, S. / Fink, E. ...Authors: Ullrich, A. / Schneider, J. / Braz, J.M. / Neu, E. / Staffen, N. / Stanek, M. / Blahova, J. / Hove, T. / Albert, T. / Allikalt, A. / Lober, S. / Bhardwaj, K. / Rodriguez-Rosado, S. / Fink, E. / Rasmussen, T. / Hubner, H. / Inoue, A. / Shoichet, B.K. / Basbaum, A.J. / Bottcher, B. / Weikert, D. / Gmeiner, P. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8pjk.cif.gz 8pjk.cif.gz | 203.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8pjk.ent.gz pdb8pjk.ent.gz | 151.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8pjk.json.gz 8pjk.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pj/8pjk https://data.pdbj.org/pub/pdb/validation_reports/pj/8pjk ftp://data.pdbj.org/pub/pdb/validation_reports/pj/8pjk ftp://data.pdbj.org/pub/pdb/validation_reports/pj/8pjk | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  17712MC  8pkmC  9gl2C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Guanine nucleotide-binding protein ... , 3 types, 3 molecules ABG
| #1: Protein | Mass: 40414.047 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNAI1 / Production host: Homo sapiens (human) / Gene: GNAI1 / Production host:  |
|---|---|
| #2: Protein | Mass: 38810.352 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNB1 / Production host: Homo sapiens (human) / Gene: GNB1 / Production host:  |
| #3: Protein | Mass: 7861.143 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: GNG2 / Production host: Homo sapiens (human) / Gene: GNG2 / Production host:  |
-Protein , 1 types, 1 molecules R
| #4: Protein | Mass: 51431.578 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: HTR1A, ADRB2RL1, ADRBRL1 / Production host: Homo sapiens (human) / Gene: HTR1A, ADRB2RL1, ADRBRL1 / Production host:  |
|---|
-Non-polymers , 4 types, 43 molecules 
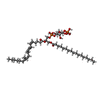



| #5: Chemical | | #6: Chemical | ChemComp-ZL9 / | Mass: 372.415 Da / Num. of mol.: 1 / Source method: obtained synthetically / Formula: C20H24N2O5 / Feature type: SUBJECT OF INVESTIGATION #7: Chemical | ChemComp-T7M / ( | #8: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: ST171-bound Serotonin 5-HT1A receptor - Gi Protein Complex Type: COMPLEX Details: Complex of human serotonin 5-HT1A receptor and Gi Protein bound to the strong partial agonist ST171 Entity ID: #1-#4 / Source: RECOMBINANT | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | |||||||||||||||||||||||||
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||||||
| Source (recombinant) | Organism:  | |||||||||||||||||||||||||
| Buffer solution | pH: 7.4 / Details: 100 mM NaCl, 20 mM HEPES, 0.002% LMNG, 0.0004 CHS | |||||||||||||||||||||||||
| Buffer component |
| |||||||||||||||||||||||||
| Specimen | Conc.: 1.9 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: This sample was monodisperse with a few impurities through dimeric complexes. | |||||||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R0.6/1 | |||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 90 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 165000 X / Nominal defocus max: 1800 nm / Nominal defocus min: 600 nm / Cs: 2.7 mm / C2 aperture diameter: 50 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 5.8 sec. / Electron dose: 80 e/Å2 / Film or detector model: FEI FALCON IV (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 14500 |
| EM imaging optics | Energyfilter name: TFS Selectris X / Energyfilter slit width: 10 eV |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 9576061 | ||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.4 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 327198 / Algorithm: FOURIER SPACE / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||
| Atomic model building | Space: REAL | ||||||||||||||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 32.71 Å2 | ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller





 PDBj
PDBj
































