[English] 日本語
 Yorodumi
Yorodumi- PDB-8k9t: Cryo-EM structure of the products-bound PGAP1(Bst1)-S327A from Ch... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8k9t | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the products-bound PGAP1(Bst1)-S327A from Chaetonium thermophilum | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / Bst1 / Glycosylphosphatidylinositol / GPI anchoring / GPI-AP / GPI-AP remodelase / Integral membrane enzyme / Lipase / Lipid remodeling / Membrane enzyme / Nanodisc / PGAP1 / TGP / Thermostable green fluorescence protein / Transmembrane enzyme / Triad enzyme. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationGPI anchor metabolic process / phosphatidylinositol deacylase activity / regulation of lipopolysaccharide-mediated signaling pathway / negative regulation of complement activation / regulation of complement-dependent cytotoxicity / regulation of complement activation / respiratory burst / positive regulation of CD4-positive, alpha-beta T cell activation / positive regulation of CD4-positive, alpha-beta T cell proliferation / Class B/2 (Secretin family receptors) ...GPI anchor metabolic process / phosphatidylinositol deacylase activity / regulation of lipopolysaccharide-mediated signaling pathway / negative regulation of complement activation / regulation of complement-dependent cytotoxicity / regulation of complement activation / respiratory burst / positive regulation of CD4-positive, alpha-beta T cell activation / positive regulation of CD4-positive, alpha-beta T cell proliferation / Class B/2 (Secretin family receptors) / ficolin-1-rich granule membrane / complement activation, classical pathway / endoplasmic reticulum to Golgi vesicle-mediated transport / transport vesicle / side of membrane / COPI-mediated anterograde transport / endoplasmic reticulum-Golgi intermediate compartment membrane / secretory granule membrane / Regulation of Complement cascade / bioluminescence / generation of precursor metabolites and energy / positive regulation of T cell cytokine production / protein transport / positive regulation of cytosolic calcium ion concentration / virus receptor activity / Hydrolases; Acting on ester bonds / membrane raft / Golgi membrane / innate immune response / Neutrophil degranulation / lipid binding / endoplasmic reticulum membrane / cell surface / extracellular exosome / extracellular region / plasma membrane Similarity search - Function | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological species |  Chaetomium thermophilum (fungus) Chaetomium thermophilum (fungus) Psychromonas sp. B3M02 (bacteria) Psychromonas sp. B3M02 (bacteria)synthetic construct (others)  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.66 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Li, T. / Hong, J. / Qu, Q. / Li, D. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  China, 1items China, 1items
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Molecular basis of the inositol deacylase PGAP1 involved in quality control of GPI-AP biogenesis. Authors: Jingjing Hong / Tingting Li / Yulin Chao / Yidan Xu / Zhini Zhu / Zixuan Zhou / Weijie Gu / Qianhui Qu / Dianfan Li /  Abstract: The secretion and quality control of glycosylphosphatidylinositol-anchored proteins (GPI-APs) necessitates post-attachment remodeling initiated by the evolutionarily conserved PGAP1, which deacylates ...The secretion and quality control of glycosylphosphatidylinositol-anchored proteins (GPI-APs) necessitates post-attachment remodeling initiated by the evolutionarily conserved PGAP1, which deacylates the inositol in nascent GPI-APs. Impairment of PGAP1 activity leads to developmental diseases in humans and fatality and infertility in animals. Here, we present three PGAP1 structures (2.66-2.84 Å), revealing its 10-transmembrane architecture and product-enzyme interaction details. PGAP1 holds GPI-AP acyl chains in an optimally organized, guitar-shaped cavity with apparent energetic penalties from hydrophobic-hydrophilic mismatches. However, abundant glycan-mediated interactions in the lumen counterbalance these repulsions, likely conferring substrate fidelity and preventing off-target hydrolysis of bulk membrane lipids. Structural and biochemical analyses uncover a serine hydrolase-type catalysis with atypical features and imply mechanisms for substrate entrance and product release involving a drawing compass movement of GPI-APs. Our findings advance the mechanistic understanding of GPI-AP remodeling. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8k9t.cif.gz 8k9t.cif.gz | 207.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8k9t.ent.gz pdb8k9t.ent.gz | 148.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8k9t.json.gz 8k9t.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/k9/8k9t https://data.pdbj.org/pub/pdb/validation_reports/k9/8k9t ftp://data.pdbj.org/pub/pdb/validation_reports/k9/8k9t ftp://data.pdbj.org/pub/pdb/validation_reports/k9/8k9t | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  36997MC  8k9qC  8k9rC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 2 types, 2 molecules AB
| #1: Protein | Mass: 161345.547 Da / Num. of mol.: 1 / Mutation: S327A,W148S,I166V,Q168Y,I202R Source method: isolated from a genetically manipulated source Details: Residues 1-1186 is GPI inositol-deacylase (Uniprot ID G0S652) with a S327A mutation. Residues 1187-1200 is the linker with a 3 C protease digestion site. Residues 1201-1435 is a fused ...Details: Residues 1-1186 is GPI inositol-deacylase (Uniprot ID G0S652) with a S327A mutation. Residues 1187-1200 is the linker with a 3 C protease digestion site. Residues 1201-1435 is a fused mCherry protein (Uniprot ID A0A366VY15 with the following four mutations that extend its fluorescence lifetime: W148S, I166V, Q168Y and I202R). Residues 1436-1447 is the linker with a His tag. Source: (gene. exp.)  Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus), (gene. exp.) Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus), (gene. exp.)  Psychromonas sp. B3M02 (bacteria) Psychromonas sp. B3M02 (bacteria)Gene: CTHT_0034210, DS885_16260 / Production host:  Homo sapiens (human) Homo sapiens (human)References: UniProt: G0S652, UniProt: A0A366VY15, Hydrolases; Acting on ester bonds |
|---|---|
| #2: Protein | Mass: 29894.154 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: Residues 1-6 is a linker. Residues 7-231 is a thermostable green fluorescent protein (PDB entry 4TZA, residue 5-229). Residues 232-263 is a linker with an expression tag. Residues 264-272 is ...Details: Residues 1-6 is a linker. Residues 7-231 is a thermostable green fluorescent protein (PDB entry 4TZA, residue 5-229). Residues 232-263 is a linker with an expression tag. Residues 264-272 is human CD55 (Uniprot ID P08174, residue P345-S353) Source: (gene. exp.) synthetic construct (others), (gene. exp.)  Homo sapiens (human) Homo sapiens (human)Gene: CD55, CR, DAF / Production host:  Homo sapiens (human) / References: UniProt: P08174 Homo sapiens (human) / References: UniProt: P08174 |
-Sugars , 2 types, 3 molecules 


| #4: Sugar | | #6: Sugar | ChemComp-PA1 / | |
|---|
-Non-polymers , 5 types, 6 molecules 
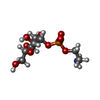







| #3: Chemical | ChemComp-PLM / | ||
|---|---|---|---|
| #5: Chemical | ChemComp-05E / | ||
| #7: Chemical | ChemComp-LYI / [( | ||
| #8: Chemical | | #9: Chemical | ChemComp-80Y / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Complex of PGAP1 with a chimera GPI-anchored protein / Type: COMPLEX / Entity ID: #1 / Source: MULTIPLE SOURCES | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.194 MDa / Experimental value: NO | ||||||||||||||||||||
| Source (natural) | Organism:  Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus) Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus) | ||||||||||||||||||||
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||
| Buffer solution | pH: 7.5 | ||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||
| Specimen | Conc.: 14.9 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid mesh size: 200 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277.15 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2500 nm / Nominal defocus min: 1200 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: FEI FALCON IV (4k x 4k) |
- Processing
Processing
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.66 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 353585 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj

























