[English] 日本語
 Yorodumi
Yorodumi- EMDB-36995: Cryo-EM structure of the GPI inositol-deacylase (PGAP1/Bst1) from... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the GPI inositol-deacylase (PGAP1/Bst1) from Chaetomium thermophilum | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Bst1 / Glycosylphosphatidylinositol / GPI anchoring / GPI-AP / GPI-AP remodelase / Integral membrane enzyme / Lipase / Lipid remodeling / Membrane enzyme / Membrane protein / Nanodisc / PGAP1 / Transmembrane enzyme / Triad enzyme | |||||||||
| Function / homology |  Function and homology information Function and homology informationGPI anchor metabolic process / phosphatidylinositol deacylase activity / endoplasmic reticulum to Golgi vesicle-mediated transport / protein transport / Hydrolases; Acting on ester bonds / endoplasmic reticulum membrane Similarity search - Function | |||||||||
| Biological species |  Thermochaetoides thermophila (fungus) / synthetic construct (others) Thermochaetoides thermophila (fungus) / synthetic construct (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.84 Å | |||||||||
 Authors Authors | Hong J / Li T / Qu Q / Li D | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Molecular basis of the inositol deacylase PGAP1 involved in quality control of GPI-AP biogenesis. Authors: Jingjing Hong / Tingting Li / Yulin Chao / Yidan Xu / Zhini Zhu / Zixuan Zhou / Weijie Gu / Qianhui Qu / Dianfan Li /  Abstract: The secretion and quality control of glycosylphosphatidylinositol-anchored proteins (GPI-APs) necessitates post-attachment remodeling initiated by the evolutionarily conserved PGAP1, which deacylates ...The secretion and quality control of glycosylphosphatidylinositol-anchored proteins (GPI-APs) necessitates post-attachment remodeling initiated by the evolutionarily conserved PGAP1, which deacylates the inositol in nascent GPI-APs. Impairment of PGAP1 activity leads to developmental diseases in humans and fatality and infertility in animals. Here, we present three PGAP1 structures (2.66-2.84 Å), revealing its 10-transmembrane architecture and product-enzyme interaction details. PGAP1 holds GPI-AP acyl chains in an optimally organized, guitar-shaped cavity with apparent energetic penalties from hydrophobic-hydrophilic mismatches. However, abundant glycan-mediated interactions in the lumen counterbalance these repulsions, likely conferring substrate fidelity and preventing off-target hydrolysis of bulk membrane lipids. Structural and biochemical analyses uncover a serine hydrolase-type catalysis with atypical features and imply mechanisms for substrate entrance and product release involving a drawing compass movement of GPI-APs. Our findings advance the mechanistic understanding of GPI-AP remodeling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36995.map.gz emd_36995.map.gz | 78.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36995-v30.xml emd-36995-v30.xml emd-36995.xml emd-36995.xml | 21.7 KB 21.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36995.png emd_36995.png | 50.9 KB | ||
| Filedesc metadata |  emd-36995.cif.gz emd-36995.cif.gz | 7.8 KB | ||
| Others |  emd_36995_half_map_1.map.gz emd_36995_half_map_1.map.gz emd_36995_half_map_2.map.gz emd_36995_half_map_2.map.gz | 77.7 MB 77.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36995 http://ftp.pdbj.org/pub/emdb/structures/EMD-36995 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36995 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36995 | HTTPS FTP |
-Related structure data
| Related structure data |  8k9qMC  8k9rC  8k9tC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_36995.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36995.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.832 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_36995_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_36995_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : GPI inositol-deacylase
| Entire | Name: GPI inositol-deacylase |
|---|---|
| Components |
|
-Supramolecule #1: GPI inositol-deacylase
| Supramolecule | Name: GPI inositol-deacylase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Thermochaetoides thermophila (fungus) Thermochaetoides thermophila (fungus) |
| Molecular weight | Theoretical: 163 KDa |
-Macromolecule #1: GPI inositol-deacylase,fused thermostable green fluorescent protein
| Macromolecule | Name: GPI inositol-deacylase,fused thermostable green fluorescent protein type: protein_or_peptide / ID: 1 Details: The protein is a fusion protein with expression tag. Residues 1187-1196 is the linker with a 3 C protease digestion site. Residues 1197-1427 is a fused thermostable green fluorescent protein ...Details: The protein is a fusion protein with expression tag. Residues 1187-1196 is the linker with a 3 C protease digestion site. Residues 1197-1427 is a fused thermostable green fluorescent protein (PDB entry 4TZA, residue 5-229). Residues 1428-1469 is the linker a Strep II tag and a His tag. Number of copies: 1 / Enantiomer: LEVO / EC number: Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 163.153094 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGSRSLSSAS SDDDDAPPIR VPRVNQCATS RTKDSQSPAQ SASKLDRRRS ADRRPSFSAN RRSGTGAGTG TGTGIANWRP FDSRDATVE RAGSSTATTA TTPPPSSSLG LMLAANGAVQ EKEMVMMGKA QEHGFVGRRA PWRSPWAISV FAFVTSLLGI G LLLAVIHS ...String: MGSRSLSSAS SDDDDAPPIR VPRVNQCATS RTKDSQSPAQ SASKLDRRRS ADRRPSFSAN RRSGTGAGTG TGTGIANWRP FDSRDATVE RAGSSTATTA TTPPPSSSLG LMLAANGAVQ EKEMVMMGKA QEHGFVGRRA PWRSPWAISV FAFVTSLLGI G LLLAVIHS SVTRQIDPKG CRMSYMRPSY AKLSDFDTEH TRLASKYSLY LYREQGIDHD VKVRGVPVLF IPGNAGSYKQ VR PIAAEAA NYFHDVLQHD EAALRAGVRS LDFFTVDFNE DITAFHGQTL LDQAEYLNEA IRYILSLYLD PRVSERDPDL PDP TSVIVL GHSMGGIVAR TMLIMPNYQH NSINTIITMS APHARPPVSF DGQIVQTYKD INNYWRHAYS QKWANDNPLW HVTL VSIAG GGLDTVVPSD YASIESLVPD THGFTVFTST IPNVWTSMDH QAILWCDQFR KVIIRALFDI VDVHRASQTK PRAQR MRVF KKWFLSGMET VAEKIAPTSD PTTLLIVDDK SDSITAEGER LVLRELGTQG SVRAHLMPIP PPGSPELKRF TLLTDT KLD KPGENGKLEV MFCSVIPSQP NPTGPAIPSQ LDLSKGNAGT TRLACTNVAP DVITLPASTR FARFPFSVRK EAEIPPF SY LEYVLDDISE HQFVAVIEKA TIPTPGFVIA EFSDHSNSHH TRHIGLRNLL TFGISLRLPS NRPMMSEVRI PSVKSSLL A YNLRISALEC SGRKDLFAPL VRQYLAEPYE SKYFVNARQA AVSLHGVAPY VPPPMSREPE AEGLAFQLWT DPTCNSSIQ VDLTVDVMGS LGKLYMRYRT VFAAFPLFIV SLVLRKQFQV YDSTGSFITF AEGLDLSLRQ SIPVMLIVLA ALTLSTTKMA PSSSAGLWH WGGNTTFTNF HQNDLLIGTQ DPFFLFLIPL IGIICVGVCT VVNYIALSLT RLISVVISFI GFLTVRFGWV N AEDRRRPS NPAIFPPSSP RRRMITTAVL LFLVSTMIPY QLAYLVACLV QLGTLVRAQR ISSELRSPAN SNFHNYVHSI FI LMLWILP INLPTLVVWM HNLSVHWLTP FTSHHNVFSI MPFILLVETH TTGQMIPRTG GTGNGRCCVL LRHITSILLL SLA LYAAVY GVSYAYTLHQ FVNLFAFWLV MVHSTADDWS LTGLRQLILH NRNNANNKSE TGSRKRGKEP GTLEVLFQGP GGSG GSASV IKPEMKIKLR MEGAVNGHKF VIEGEGIGKP YEGTQTLDLT VEEGAPLPFS YDILTPAFQY GNRAFTKYPE DIPDY FKQA FPEGYSWERS MTYEDQGICI ATSDITMEGD CFFYEIRFDG TNFPPNGPVM QKKTLKWEPS TEKMYVEDGV LKGDVE MAL LLEGGGHYRC DFKTTYKAKK DVRLPDAHEV DHRIEILSHD KDYNKVRLYE HAEARYSGGG SGGGSAWSHP QFEKGGG SG GGSGGSAWSH PQFEKGSHHH HHHHHHH UniProtKB: GPI inositol-deacylase |
-Macromolecule #2: CHOLESTEROL HEMISUCCINATE
| Macromolecule | Name: CHOLESTEROL HEMISUCCINATE / type: ligand / ID: 2 / Number of copies: 3 / Formula: Y01 |
|---|---|
| Molecular weight | Theoretical: 486.726 Da |
| Chemical component information |  ChemComp-Y01: |
-Macromolecule #3: (2~{S})-2-azanyl-3-[[(2~{R})-3-hexadecanoyloxy-2-[(~{Z})-octadec-...
| Macromolecule | Name: (2~{S})-2-azanyl-3-[[(2~{R})-3-hexadecanoyloxy-2-[(~{Z})-octadec-9-enoyl]oxy-propoxy]-oxidanyl-phosphoryl]oxy-propanoic acid type: ligand / ID: 3 / Number of copies: 2 / Formula: D39 |
|---|---|
| Molecular weight | Theoretical: 762.006 Da |
| Chemical component information | 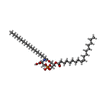 ChemComp-D39: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 7.5 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 50 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.026000000000000002 kPa | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV / Details: Blot for 4.5s before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




































