+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7uwl | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of the IL-25-IL-17RB-IL-17RA ternary complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords | CYTOKINE / Receptor complex / IL-17E / IL-25 / IL-17RB / IL-17RA | ||||||
| Function / homology |  Function and homology information Function and homology informationinterleukin-17E receptor binding / interleukin-17 receptor activity / eosinophil differentiation / granulocyte chemotaxis / Interleukin-17 signaling / T-helper 17 type immune response / interleukin-17A-mediated signaling pathway / positive regulation of interleukin-23 production / positive regulation of chemokine (C-X-C motif) ligand 1 production / response to nematode ...interleukin-17E receptor binding / interleukin-17 receptor activity / eosinophil differentiation / granulocyte chemotaxis / Interleukin-17 signaling / T-helper 17 type immune response / interleukin-17A-mediated signaling pathway / positive regulation of interleukin-23 production / positive regulation of chemokine (C-X-C motif) ligand 1 production / response to nematode / response to fungus / interleukin-17-mediated signaling pathway / positive regulation of interleukin-13 production / positive regulation of interleukin-5 production / fibroblast activation / positive regulation of cytokine production involved in inflammatory response / inflammatory response to antigenic stimulus / cytokine receptor activity / defense response to fungus / cytokine activity / regulation of cell growth / response to virus / protein catabolic process / defense response / positive regulation of inflammatory response / positive regulation of interleukin-6 production / cell surface receptor signaling pathway / inflammatory response / intracellular membrane-bounded organelle / innate immune response / signaling receptor binding / SARS-CoV-2 activates/modulates innate and adaptive immune responses / positive regulation of transcription by RNA polymerase II / extracellular space / extracellular region / membrane / plasma membrane Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.7 Å | ||||||
 Authors Authors | Wilson, S.C. / Caveney, N.A. / Jude, K.M. / Garcia, K.C. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Organizing structural principles of the IL-17 ligand-receptor axis. Authors: Steven C Wilson / Nathanael A Caveney / Michelle Yen / Christoph Pollmann / Xinyu Xiang / Kevin M Jude / Maximillian Hafer / Naotaka Tsutsumi / Jacob Piehler / K Christopher Garcia /   Abstract: The IL-17 family of cytokines and receptors have central roles in host defence against infection and development of inflammatory diseases. The compositions and structures of functional IL-17 family ...The IL-17 family of cytokines and receptors have central roles in host defence against infection and development of inflammatory diseases. The compositions and structures of functional IL-17 family ligand-receptor signalling assemblies remain unclear. IL-17E (also known as IL-25) is a key regulator of type 2 immune responses and driver of inflammatory diseases, such as allergic asthma, and requires both IL-17 receptor A (IL-17RA) and IL-17RB to elicit functional responses. Here we studied IL-25-IL-17RB binary and IL-25-IL-17RB-IL-17RA ternary complexes using a combination of cryo-electron microscopy, single-molecule imaging and cell-based signalling approaches. The IL-25-IL-17RB-IL-17RA ternary signalling assembly is a C2-symmetric complex in which the IL-25-IL-17RB homodimer is flanked by two 'wing-like' IL-17RA co-receptors through a 'tip-to-tip' geometry that is the key receptor-receptor interaction required for initiation of signal transduction. IL-25 interacts solely with IL-17RB to allosterically promote the formation of the IL-17RB-IL-17RA tip-to-tip interface. The resulting large separation between the receptors at the membrane-proximal level may reflect proximity constraints imposed by the intracellular domains for signalling. Cryo-electron microscopy structures of IL-17A-IL-17RA and IL-17A-IL-17RA-IL-17RC complexes reveal that this tip-to-tip architecture is a key organizing principle of the IL-17 receptor family. Furthermore, these studies reveal dual actions for IL-17RA sharing among IL-17 cytokine complexes, by either directly engaging IL-17 cytokines or alternatively functioning as a co-receptor. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7uwl.cif.gz 7uwl.cif.gz | 242 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7uwl.ent.gz pdb7uwl.ent.gz | 189.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7uwl.json.gz 7uwl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7uwl_validation.pdf.gz 7uwl_validation.pdf.gz | 1.6 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7uwl_full_validation.pdf.gz 7uwl_full_validation.pdf.gz | 1.7 MB | Display | |
| Data in XML |  7uwl_validation.xml.gz 7uwl_validation.xml.gz | 58.3 KB | Display | |
| Data in CIF |  7uwl_validation.cif.gz 7uwl_validation.cif.gz | 83.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/uw/7uwl https://data.pdbj.org/pub/pdb/validation_reports/uw/7uwl ftp://data.pdbj.org/pub/pdb/validation_reports/uw/7uwl ftp://data.pdbj.org/pub/pdb/validation_reports/uw/7uwl | HTTPS FTP |
-Related structure data
| Related structure data |  26835MC  7uwjC  7uwkC  7uwmC  7uwnC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 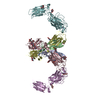
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 21490.201 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: IL25, IL17E, UNQ3120/PRO10272 / Production host: Homo sapiens (human) / Gene: IL25, IL17E, UNQ3120/PRO10272 / Production host:  Homo sapiens (human) / References: UniProt: Q9H293 Homo sapiens (human) / References: UniProt: Q9H293#2: Protein | Mass: 33649.098 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: IL17RB, CRL4, EVI27, IL17BR, UNQ2501/PRO19612 / Production host: Homo sapiens (human) / Gene: IL17RB, CRL4, EVI27, IL17BR, UNQ2501/PRO19612 / Production host:  Homo sapiens (human) / References: UniProt: Q9NRM6 Homo sapiens (human) / References: UniProt: Q9NRM6#3: Protein | Mass: 36888.617 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: IL17RA, IL17R / Production host: Homo sapiens (human) / Gene: IL17RA, IL17R / Production host:  Homo sapiens (human) / References: UniProt: Q96F46 Homo sapiens (human) / References: UniProt: Q96F46#4: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #5: Sugar | ChemComp-NAG / Has ligand of interest | N | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: ternary IL-25-IL-17RB-IL-17RA complex / Type: COMPLEX / Entity ID: #1-#3 / Source: RECOMBINANT |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER |
| Electron lens | Mode: OTHER / Nominal defocus max: 2200 nm / Nominal defocus min: 800 nm |
| Image recording | Electron dose: 53 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.20.1_4487: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Symmetry | Point symmetry: C2 (2 fold cyclic) | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.7 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 1287497 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller







 PDBj
PDBj



