[English] 日本語
 Yorodumi
Yorodumi- PDB-7uhb: SARS-CoV-2 spike in complex with AHB2-2GS-SB175 (local refinement... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7uhb | ||||||
|---|---|---|---|---|---|---|---|
| Title | SARS-CoV-2 spike in complex with AHB2-2GS-SB175 (local refinement of the RBD and AHB2) | ||||||
 Components Components |
| ||||||
 Keywords Keywords | VIRAL PROTEIN / SARS-CoV-2 / COVID-19 / spike glycoprotein / fusion protein / neutralizing antibodies / Structural Genomics / Seattle Structural Genomics Center for Infectious Disease / SSGCID / Inhibitor | ||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion ...symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / viral translation / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion / entry receptor-mediated virion attachment to host cell / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | ||||||
| Biological species |  Severe acute respiratory syndrome coronavirus Severe acute respiratory syndrome coronavirussynthetic construct (others) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3 Å | ||||||
 Authors Authors | Park, Y.J. / Seattle Structural Genomics Center for Infectious Disease (SSGCID) / Veesler, D. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Sci Transl Med / Year: 2022 Journal: Sci Transl Med / Year: 2022Title: Multivalent designed proteins neutralize SARS-CoV-2 variants of concern and confer protection against infection in mice. Authors: Andrew C Hunt / James Brett Case / Young-Jun Park / Longxing Cao / Kejia Wu / Alexandra C Walls / Zhuoming Liu / John E Bowen / Hsien-Wei Yeh / Shally Saini / Louisa Helms / Yan Ting Zhao / ...Authors: Andrew C Hunt / James Brett Case / Young-Jun Park / Longxing Cao / Kejia Wu / Alexandra C Walls / Zhuoming Liu / John E Bowen / Hsien-Wei Yeh / Shally Saini / Louisa Helms / Yan Ting Zhao / Tien-Ying Hsiang / Tyler N Starr / Inna Goreshnik / Lisa Kozodoy / Lauren Carter / Rashmi Ravichandran / Lydia B Green / Wadim L Matochko / Christy A Thomson / Bastian Vögeli / Antje Krüger / Laura A VanBlargan / Rita E Chen / Baoling Ying / Adam L Bailey / Natasha M Kafai / Scott E Boyken / Ajasja Ljubetič / Natasha Edman / George Ueda / Cameron M Chow / Max Johnson / Amin Addetia / Mary-Jane Navarro / Nuttada Panpradist / Michael Gale / Benjamin S Freedman / Jesse D Bloom / Hannele Ruohola-Baker / Sean P J Whelan / Lance Stewart / Michael S Diamond / David Veesler / Michael C Jewett / David Baker /    Abstract: New variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continue to arise and prolong the coronavirus disease 2019 (COVID-19) pandemic. Here, we used a cell-free expression ...New variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continue to arise and prolong the coronavirus disease 2019 (COVID-19) pandemic. Here, we used a cell-free expression workflow to rapidly screen and optimize constructs containing multiple computationally designed miniprotein inhibitors of SARS-CoV-2. We found the broadest efficacy was achieved with a homotrimeric version of the 75-residue angiotensin-converting enzyme 2 (ACE2) mimic AHB2 (TRI2-2) designed to geometrically match the trimeric spike architecture. Consistent with the design model, in the cryo-electron microscopy structure TRI2-2 forms a tripod at the apex of the spike protein that engaged all three receptor binding domains simultaneously. TRI2-2 neutralized Omicron (B.1.1.529), Delta (B.1.617.2), and all other variants tested with greater potency than the monoclonal antibodies used clinically for the treatment of COVID-19. TRI2-2 also conferred prophylactic and therapeutic protection against SARS-CoV-2 challenge when administered intranasally in mice. Designed miniprotein receptor mimics geometrically arrayed to match pathogen receptor binding sites could be a widely applicable antiviral therapeutic strategy with advantages over antibodies in greater resistance to viral escape and antigenic drift, and advantages over native receptor traps in lower chances of autoimmune responses. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7uhb.cif.gz 7uhb.cif.gz | 92.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7uhb.ent.gz pdb7uhb.ent.gz | 53.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7uhb.json.gz 7uhb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/uh/7uhb https://data.pdbj.org/pub/pdb/validation_reports/uh/7uhb ftp://data.pdbj.org/pub/pdb/validation_reports/uh/7uhb ftp://data.pdbj.org/pub/pdb/validation_reports/uh/7uhb | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  26511MC  7uhcC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 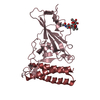
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 142427.438 Da / Num. of mol.: 1 / Mutation: R682,R683,R685,F817,A892,A899,A942,K986,V987 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Severe acute respiratory syndrome coronavirus Severe acute respiratory syndrome coronavirusGene: S, 2 / Production host:  Homo sapiens (human) / References: UniProt: P0DTC2 Homo sapiens (human) / References: UniProt: P0DTC2 |
|---|---|
| #2: Protein | Mass: 17565.787 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.) synthetic construct (others) / Production host:  |
| #3: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
| Has ligand of interest | N |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | ||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||
| Buffer solution | pH: 8 | ||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2500 nm / Nominal defocus min: 500 nm / Cs: 2.7 mm |
| Image recording | Electron dose: 60 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| EM software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||
| 3D reconstruction | Resolution: 3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 206541 / Symmetry type: POINT |
 Movie
Movie Controller
Controller







 PDBj
PDBj




