[English] 日本語
 Yorodumi
Yorodumi- EMDB-4481: Complex III2 focused refinement from Ovine respiratory supercompl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4481 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Complex III2 focused refinement from Ovine respiratory supercomplex I+III2 | |||||||||
 Map data Map data | Focused refinement around complex III2 from ovine mitochondrial supercomplex I III2 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | complex III / cellular respiration / mitochondria / ELECTRON TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology information: / : / : / respiratory chain complex III / quinol-cytochrome-c reductase / ubiquinol-cytochrome-c reductase activity / mitochondrial electron transport, ubiquinol to cytochrome c / membrane => GO:0016020 / metalloendopeptidase activity / 2 iron, 2 sulfur cluster binding ...: / : / : / respiratory chain complex III / quinol-cytochrome-c reductase / ubiquinol-cytochrome-c reductase activity / mitochondrial electron transport, ubiquinol to cytochrome c / membrane => GO:0016020 / metalloendopeptidase activity / 2 iron, 2 sulfur cluster binding / electron transfer activity / mitochondrial inner membrane / ubiquitin protein ligase binding / heme binding / proteolysis / nucleoplasm / metal ion binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
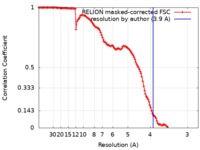
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Letts JA / Sazanov LA | |||||||||
| Funding support |  Austria, 1 items Austria, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2019 Journal: Mol Cell / Year: 2019Title: Structures of Respiratory Supercomplex I+III Reveal Functional and Conformational Crosstalk. Authors: James A Letts / Karol Fiedorczuk / Gianluca Degliesposti / Mark Skehel / Leonid A Sazanov /    Abstract: The mitochondrial electron transport chain complexes are organized into supercomplexes (SCs) of defined stoichiometry, which have been proposed to regulate electron flux via substrate channeling. We ...The mitochondrial electron transport chain complexes are organized into supercomplexes (SCs) of defined stoichiometry, which have been proposed to regulate electron flux via substrate channeling. We demonstrate that CoQ trapping in the isolated SC I+III limits complex (C)I turnover, arguing against channeling. The SC structure, resolved at up to 3.8 Å in four distinct states, suggests that CoQ oxidation may be rate limiting because of unequal access of CoQ to the active sites of CIII. CI shows a transition between "closed" and "open" conformations, accompanied by the striking rotation of a key transmembrane helix. Furthermore, the state of CI affects the conformational flexibility within CIII, demonstrating crosstalk between the enzymes. CoQ was identified at only three of the four binding sites in CIII, suggesting that interaction with CI disrupts CIII symmetry in a functionally relevant manner. Together, these observations indicate a more nuanced functional role for the SCs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4481.map.gz emd_4481.map.gz | 172.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4481-v30.xml emd-4481-v30.xml emd-4481.xml emd-4481.xml | 30.5 KB 30.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4481_fsc.xml emd_4481_fsc.xml | 12.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_4481.png emd_4481.png | 92.7 KB | ||
| Masks |  emd_4481_msk_1.map emd_4481_msk_1.map | 184 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-4481.cif.gz emd-4481.cif.gz | 8.3 KB | ||
| Others |  emd_4481_half_map_1.map.gz emd_4481_half_map_1.map.gz emd_4481_half_map_2.map.gz emd_4481_half_map_2.map.gz | 145.3 MB 145.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4481 http://ftp.pdbj.org/pub/emdb/structures/EMD-4481 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4481 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4481 | HTTPS FTP |
-Validation report
| Summary document |  emd_4481_validation.pdf.gz emd_4481_validation.pdf.gz | 894.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_4481_full_validation.pdf.gz emd_4481_full_validation.pdf.gz | 894.2 KB | Display | |
| Data in XML |  emd_4481_validation.xml.gz emd_4481_validation.xml.gz | 20.1 KB | Display | |
| Data in CIF |  emd_4481_validation.cif.gz emd_4481_validation.cif.gz | 26.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4481 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4481 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4481 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4481 | HTTPS FTP |
-Related structure data
| Related structure data |  6q9eMC  4479C  4480C  4482C  4493C  4494C  4495C  4496C  4497C  4498C  4499C  4500C  4501C  4502C  4505C  4506C  4507C  6q9bC  6q9dC  6qa9C  6qbxC  6qc2C  6qc3C  6qc4C  6qc5C  6qc6C  6qc7C  6qc8C  6qc9C  6qcaC  6qcfC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4481.map.gz / Format: CCP4 / Size: 184 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4481.map.gz / Format: CCP4 / Size: 184 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused refinement around complex III2 from ovine mitochondrial supercomplex I III2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.4 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
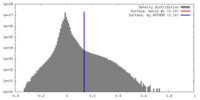
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_4481_msk_1.map emd_4481_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
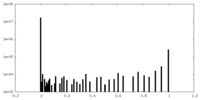
| Density Histograms |
-Half map: Focused refinement around complex III2 from ovine mitochondrial...
| File | emd_4481_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused refinement around complex III2 from ovine mitochondrial supercomplex I III2. Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
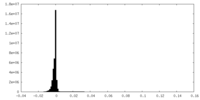
| Density Histograms |
-Half map: Focused refinement around complex III2 from ovine mitochondrial...
| File | emd_4481_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Focused refinement around complex III2 from ovine mitochondrial supercomplex I III2. Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
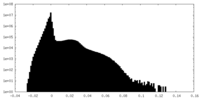
| Density Histograms |
- Sample components
Sample components
+Entire : Ovine mitochondrial SC I+III2
+Supramolecule #1: Ovine mitochondrial SC I+III2
+Macromolecule #1: Ubiquinol-cytochrome c reductase core protein 1
+Macromolecule #2: Ubiquinol-cytochrome c reductase core protein 2
+Macromolecule #3: Cytochrome b
+Macromolecule #4: Cytochrome c1
+Macromolecule #5: Cytochrome b-c1 complex subunit Rieske, mitochondrial
+Macromolecule #6: Cytochrome b-c1 complex subunit 7
+Macromolecule #7: Ubiquinol-cytochrome c reductase complex III subunit VII
+Macromolecule #8: Cytochrome b-c1 complex subunit 6
+Macromolecule #9: Cytochrome b-c1 complex subunit Rieske, mitochondrial
+Macromolecule #10: Ubiquinol-cytochrome c reductase, complex III subunit X
+Macromolecule #11: PROTOPORPHYRIN IX CONTAINING FE
+Macromolecule #12: 1,2-Distearoyl-sn-glycerophosphoethanolamine
+Macromolecule #13: CARDIOLIPIN
+Macromolecule #14: UBIQUINONE-10
+Macromolecule #15: HEME C
+Macromolecule #16: FE2/S2 (INORGANIC) CLUSTER
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: 250 mM NaCl, 20 mM HEPES, pH 7.7, 0.02% Brij-35 | ||||||||||||
| Vitrification | Cryogen name: PROPANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: blotting for 30 seconds at 4 degrees Celsius, 95% humidity and flash freezing. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Number grids imaged: 1 / Number real images: 1854 / Average exposure time: 2.0 sec. / Average electron dose: 51.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)