+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human OGG1 bound to a 35-bp DNA with an 8-oxoG in the middle | |||||||||
 Map data Map data | Human OGG1 binding at 8-oxoG on a 35 bp DNA half map 1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Human OGG1 binding at 8-oxoG of a 35 bp DNA duplex / HYDROLASE / LYASE-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationDefective OGG1 Substrate Binding / Defective OGG1 Substrate Processing / Defective OGG1 Localization / depurination / negative regulation of double-strand break repair via single-strand annealing / oxidized purine nucleobase lesion DNA N-glycosylase activity / base-excision repair, AP site formation / depyrimidination / 8-oxo-7,8-dihydroguanine DNA N-glycosylase activity / Displacement of DNA glycosylase by APEX1 ...Defective OGG1 Substrate Binding / Defective OGG1 Substrate Processing / Defective OGG1 Localization / depurination / negative regulation of double-strand break repair via single-strand annealing / oxidized purine nucleobase lesion DNA N-glycosylase activity / base-excision repair, AP site formation / depyrimidination / 8-oxo-7,8-dihydroguanine DNA N-glycosylase activity / Displacement of DNA glycosylase by APEX1 / positive regulation of gene expression via chromosomal CpG island demethylation / Hydrolases; Glycosylases; Hydrolysing N-glycosyl compounds / oxidized purine DNA binding / APEX1-Independent Resolution of AP Sites via the Single Nucleotide Replacement Pathway / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / class I DNA-(apurinic or apyrimidinic site) endonuclease activity / DNA-(apurinic or apyrimidinic site) lyase / cellular response to reactive oxygen species / nucleotide-excision repair / response to radiation / base-excision repair / nuclear matrix / response to oxidative stress / endonuclease activity / microtubule binding / damaged DNA binding / nuclear speck / RNA polymerase II cis-regulatory region sequence-specific DNA binding / mitochondrial matrix / DNA damage response / regulation of DNA-templated transcription / enzyme binding / positive regulation of transcription by RNA polymerase II / protein-containing complex / mitochondrion / DNA binding / nucleoplasm / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | You Q / Li H | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2024 Journal: Commun Biol / Year: 2024Title: Human 8-oxoguanine glycosylase OGG1 binds nucleosome at the dsDNA ends and the super-helical locations. Authors: Qinglong You / Xiang Feng / Yi Cai / Stephen B Baylin / Huilin Li /  Abstract: The human glycosylase OGG1 extrudes and excises the oxidized DNA base 8-oxoguanine (8-oxoG) to initiate base excision repair and plays important roles in many pathological conditions such as cancer, ...The human glycosylase OGG1 extrudes and excises the oxidized DNA base 8-oxoguanine (8-oxoG) to initiate base excision repair and plays important roles in many pathological conditions such as cancer, inflammation, and neurodegenerative diseases. Previous structural studies have used a truncated protein and short linear DNA, so it has been unclear how full-length OGG1 operates on longer DNA or on nucleosomes. Here we report cryo-EM structures of human OGG1 bound to a 35-bp long DNA containing an 8-oxoG within an unmethylated Cp-8-oxoG dinucleotide as well as to a nucleosome with an 8-oxoG at super-helical location (SHL)-5. The 8-oxoG in the linear DNA is flipped out by OGG1, consistent with previous crystallographic findings with a 15-bp DNA. OGG1 preferentially binds near dsDNA ends at the nucleosomal entry/exit sites. Such preference may underlie the enzyme's function in DNA double-strand break repair. Unexpectedly, we find that OGG1 bends the nucleosomal entry DNA, flips an undamaged guanine, and binds to internal nucleosomal DNA sites such as SHL-5 and SHL+6. We suggest that the DNA base search mechanism by OGG1 may be chromatin context-dependent and that OGG1 may partner with chromatin remodelers to excise 8-oxoG at the nucleosomal internal sites. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43607.map.gz emd_43607.map.gz | 3.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43607-v30.xml emd-43607-v30.xml emd-43607.xml emd-43607.xml | 18.6 KB 18.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_43607.png emd_43607.png | 67.6 KB | ||
| Filedesc metadata |  emd-43607.cif.gz emd-43607.cif.gz | 6.3 KB | ||
| Others |  emd_43607_half_map_1.map.gz emd_43607_half_map_1.map.gz emd_43607_half_map_2.map.gz emd_43607_half_map_2.map.gz | 23.1 MB 23.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43607 http://ftp.pdbj.org/pub/emdb/structures/EMD-43607 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43607 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43607 | HTTPS FTP |
-Related structure data
| Related structure data |  8vx4MC  8vx5C  8vx6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_43607.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43607.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Human OGG1 binding at 8-oxoG on a 35 bp DNA half map 1 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.828 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Human OGG1 binding at 8-oxoG on a 35 bp DNA
| File | emd_43607_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Human OGG1 binding at 8-oxoG on a 35 bp DNA | ||||||||||||
| Projections & Slices |
| ||||||||||||
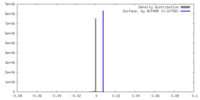
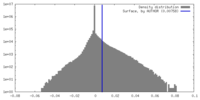
| Density Histograms |
-Half map: Human OGG1 binding at 8-oxoG on a 35 bp DNA half map 2
| File | emd_43607_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Human OGG1 binding at 8-oxoG on a 35 bp DNA half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
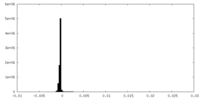
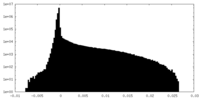
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of Human OGG1 with a 35 bp DNA duplex containing 8-oxoG
| Entire | Name: Complex of Human OGG1 with a 35 bp DNA duplex containing 8-oxoG |
|---|---|
| Components |
|
-Supramolecule #1: Complex of Human OGG1 with a 35 bp DNA duplex containing 8-oxoG
| Supramolecule | Name: Complex of Human OGG1 with a 35 bp DNA duplex containing 8-oxoG type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: DNA (35-MER)
| Macromolecule | Name: DNA (35-MER) / type: dna / ID: 1 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 10.695851 KDa |
| Sequence | String: (DA)(DT)(DG)(DC)(DC)(DT)(DC)(DG)(DC)(DA) (DG)(DA)(DA)(DT)(DC)(DC)(DC)(8OG)(DC) (DT)(DG)(DC)(DC)(DG)(DA)(DG)(DG)(DC)(DC) (DG)(DC)(DT)(DC)(DA)(DA) |
-Macromolecule #2: DNA (35-MER)
| Macromolecule | Name: DNA (35-MER) / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 10.861943 KDa |
| Sequence | String: (DT)(DT)(DG)(DA)(DG)(DC)(DG)(DG)(DC)(DC) (DT)(DC)(DG)(DG)(DC)(DA)(DG)(DC)(DG)(DG) (DG)(DA)(DT)(DT)(DC)(DT)(DG)(DC)(DG) (DA)(DG)(DG)(DC)(DA)(DT) |
-Macromolecule #3: N-glycosylase/DNA lyase
| Macromolecule | Name: N-glycosylase/DNA lyase / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Glycosylases; Hydrolysing N-glycosyl compounds |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 43.72002 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGHHHHHHDY KDHDGDYKDH DIDYKDDDDK ENLYFQGGGG GSDPARALLP RRMGHRTLAS TPALWASIPC PRSELRLDLV LPSGQSFRW REQSPAHWSG VLADQVWTLT QTEEQLHCTV YRGDKSQASR PTPDELEAVR KYFQLDVTLA QLYHHWGSVD S HFQEVAQK ...String: MGHHHHHHDY KDHDGDYKDH DIDYKDDDDK ENLYFQGGGG GSDPARALLP RRMGHRTLAS TPALWASIPC PRSELRLDLV LPSGQSFRW REQSPAHWSG VLADQVWTLT QTEEQLHCTV YRGDKSQASR PTPDELEAVR KYFQLDVTLA QLYHHWGSVD S HFQEVAQK FQGVRLLRQD PIECLFSFIC SSNNNIARIT GMVERLCQAF GPRLIQLDDV TYHGFPSLQA LAGPEVEAHL RK LGLGYRA RYVSASARAI LEEQGGLAWL QQLRESSYEE AHKALCILPG VGTQVADCIC LMALDKPQAV PVDVHMWHIA QRD YSWHPT TSQAKGPSPQ TNKELGNFFR SLWGPYAGWA QAVLFSADLR QSRHAQEPPA KRRKGSKGPE G UniProtKB: N-glycosylase/DNA lyase |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 64.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-8vx4: |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)