+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of GATA4 in complex with ALBN1 nucleosome | |||||||||
 Map data Map data | main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | nucleosome / pioneer transcription factors / DNA binding proteins / transcription / chromatin / NUCLEAR PROTEIN / NUCLEAR PROTEIN-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationatrioventricular valve formation / atrial septum secundum morphogenesis / embryonic heart tube anterior/posterior pattern specification / transdifferentiation / Formation of lateral plate mesoderm / intestinal epithelial cell differentiation / cardiac right ventricle morphogenesis / atrioventricular node development / co-SMAD binding / atrial septum morphogenesis ...atrioventricular valve formation / atrial septum secundum morphogenesis / embryonic heart tube anterior/posterior pattern specification / transdifferentiation / Formation of lateral plate mesoderm / intestinal epithelial cell differentiation / cardiac right ventricle morphogenesis / atrioventricular node development / co-SMAD binding / atrial septum morphogenesis / cardiac muscle tissue regeneration / cell growth involved in cardiac muscle cell development / Transcriptional regulation of testis differentiation / cardiac ventricle morphogenesis / endocardial cushion development / atrial septum primum morphogenesis / Physiological factors / atrioventricular canal development / embryonic foregut morphogenesis / YAP1- and WWTR1 (TAZ)-stimulated gene expression / Synthesis, secretion, and inactivation of Glucose-dependent Insulinotropic Polypeptide (GIP) / Formation of definitive endoderm / endoderm development / positive regulation of BMP signaling pathway / Developmental Lineage of Pancreatic Acinar Cells / response to vitamin A / regulation of cardiac muscle cell contraction / Cardiogenesis / aortic valve morphogenesis / negative regulation of cardiac muscle cell apoptotic process / ventricular septum development / DNA-binding transcription activator activity / NFAT protein binding / heart looping / detection of maltose stimulus / maltose transport complex / carbohydrate transport / positive regulation of vascular endothelial growth factor production / negative regulation of apoptotic signaling pathway / carbohydrate transmembrane transporter activity / maltose binding / cell fate commitment / maltose transport / maltodextrin transmembrane transport / negative regulation of tumor necrosis factor-mediated signaling pathway / response to mechanical stimulus / negative regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / negative regulation of megakaryocyte differentiation / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / Packaging Of Telomere Ends / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / telomere organization / negative regulation of autophagy / Interleukin-7 signaling / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / ATP-binding cassette (ABC) transporter complex / RNA Polymerase I Promoter Opening / epigenetic regulation of gene expression / Inhibition of DNA recombination at telomere / Assembly of the ORC complex at the origin of replication / Meiotic synapsis / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / cell chemotaxis / Regulation of endogenous retroelements by KRAB-ZFP proteins / RNA polymerase II transcription regulatory region sequence-specific DNA binding / innate immune response in mucosa / Defective pyroptosis / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / RNA Polymerase I Promoter Escape / Nonhomologous End-Joining (NHEJ) / wound healing / cellular response to glucose stimulus / lipopolysaccharide binding / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / HDMs demethylate histones / G2/M DNA damage checkpoint / NoRC negatively regulates rRNA expression / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / DNA Damage/Telomere Stress Induced Senescence / Pre-NOTCH Transcription and Translation Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
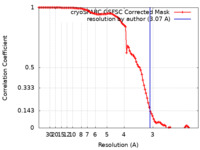
| Method | single particle reconstruction / cryo EM / Resolution: 3.07 Å | |||||||||
 Authors Authors | Zhou BR / Bai Y | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2024 Journal: Mol Cell / Year: 2024Title: Structural insights into the cooperative nucleosome recognition and chromatin opening by FOXA1 and GATA4. Authors: Bing-Rui Zhou / Hanqiao Feng / Furong Huang / Iris Zhu / Stephanie Portillo-Ledesma / Dan Shi / Kenneth S Zaret / Tamar Schlick / David Landsman / Qianben Wang / Yawen Bai /   Abstract: Mouse FOXA1 and GATA4 are prototypes of pioneer factors, initiating liver cell development by binding to the N1 nucleosome in the enhancer of the ALB1 gene. Using cryoelectron microscopy (cryo-EM), ...Mouse FOXA1 and GATA4 are prototypes of pioneer factors, initiating liver cell development by binding to the N1 nucleosome in the enhancer of the ALB1 gene. Using cryoelectron microscopy (cryo-EM), we determined the structures of the free N1 nucleosome and its complexes with FOXA1 and GATA4, both individually and in combination. We found that the DNA-binding domains of FOXA1 and GATA4 mainly recognize the linker DNA and an internal site in the nucleosome, respectively, whereas their intrinsically disordered regions interact with the acidic patch on histone H2A-H2B. FOXA1 efficiently enhances GATA4 binding by repositioning the N1 nucleosome. In vivo DNA editing and bioinformatics analyses suggest that the co-binding mode of FOXA1 and GATA4 plays important roles in regulating genes involved in liver cell functions. Our results reveal the mechanism whereby FOXA1 and GATA4 cooperatively bind to the nucleosome through nucleosome repositioning, opening chromatin by bending linker DNA and obstructing nucleosome packing. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43196.map.gz emd_43196.map.gz | 53.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43196-v30.xml emd-43196-v30.xml emd-43196.xml emd-43196.xml | 26.3 KB 26.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_43196_fsc.xml emd_43196_fsc.xml | 9.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_43196.png emd_43196.png | 156.4 KB | ||
| Filedesc metadata |  emd-43196.cif.gz emd-43196.cif.gz | 7.8 KB | ||
| Others |  emd_43196_half_map_1.map.gz emd_43196_half_map_1.map.gz emd_43196_half_map_2.map.gz emd_43196_half_map_2.map.gz | 95.6 MB 95.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43196 http://ftp.pdbj.org/pub/emdb/structures/EMD-43196 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43196 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43196 | HTTPS FTP |
-Related structure data
| Related structure data |  8vg0MC  8vfxC  8vfyC  8vfzC  8vg1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_43196.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43196.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map | ||||||||||||||||||||||||||||||||||||
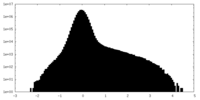
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.056 Å | ||||||||||||||||||||||||||||||||||||
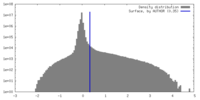
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half map 1
| File | emd_43196_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
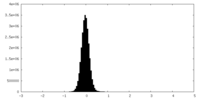
| Density Histograms |
-Half map: half map 2
| File | emd_43196_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
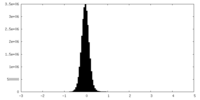
| Density Histograms |
- Sample components
Sample components
-Entire : GATA4 in complex with 186bp ALBN1 nucleosome
| Entire | Name: GATA4 in complex with 186bp ALBN1 nucleosome |
|---|---|
| Components |
|
-Supramolecule #1: GATA4 in complex with 186bp ALBN1 nucleosome
| Supramolecule | Name: GATA4 in complex with 186bp ALBN1 nucleosome / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#7 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: DNA (159-MER)
| Macromolecule | Name: DNA (159-MER) / type: dna / ID: 1 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 57.399637 KDa |
| Sequence | String: (DA)(DT)(DC)(DC)(DG)(DA)(DG)(DA)(DT)(DG) (DG)(DT)(DA)(DC)(DT)(DT)(DT)(DG)(DT)(DG) (DT)(DC)(DT)(DC)(DC)(DT)(DG)(DC)(DT) (DC)(DT)(DG)(DT)(DC)(DA)(DG)(DC)(DA)(DG) (DG) (DG)(DC)(DA)(DC)(DT)(DG) ...String: (DA)(DT)(DC)(DC)(DG)(DA)(DG)(DA)(DT)(DG) (DG)(DT)(DA)(DC)(DT)(DT)(DT)(DG)(DT)(DG) (DT)(DC)(DT)(DC)(DC)(DT)(DG)(DC)(DT) (DC)(DT)(DG)(DT)(DC)(DA)(DG)(DC)(DA)(DG) (DG) (DG)(DC)(DA)(DC)(DT)(DG)(DT)(DA) (DC)(DT)(DT)(DG)(DC)(DT)(DG)(DA)(DT)(DA) (DC)(DC) (DA)(DG)(DG)(DG)(DA)(DA)(DT) (DG)(DT)(DT)(DT)(DG)(DT)(DT)(DC)(DT)(DT) (DA)(DA)(DA) (DT)(DA)(DC)(DC)(DA)(DT) (DC)(DA)(DT)(DT)(DC)(DC)(DG)(DG)(DA)(DC) (DG)(DT)(DG)(DT) (DT)(DT)(DG)(DC)(DC) (DT)(DT)(DG)(DG)(DC)(DC)(DA)(DG)(DT)(DT) (DT)(DT)(DC)(DC)(DA) (DT)(DG)(DT)(DA) (DC)(DA)(DT)(DG)(DC)(DA)(DG)(DA)(DA)(DA) (DG)(DA)(DA)(DG)(DT)(DT) (DT)(DG)(DG) (DA)(DC)(DT)(DG)(DA)(DT)(DC)(DA)(DA)(DT) (DA)(DC)(DA)(DG)(DT)(DC)(DC) (DT)(DC) (DT)(DG)(DC)(DC)(DT)(DT)(DT)(DA)(DA)(DA) (DG)(DC)(DA)(DA)(DT)(DA)(DG)(DG) (DA) (DA)(DA)(DG)(DA)(DT) |
-Macromolecule #2: DNA (159-MER)
| Macromolecule | Name: DNA (159-MER) / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 57.42777 KDa |
| Sequence | String: (DA)(DT)(DC)(DT)(DT)(DT)(DC)(DC)(DT)(DA) (DT)(DT)(DG)(DC)(DT)(DT)(DT)(DA)(DA)(DA) (DG)(DG)(DC)(DA)(DG)(DA)(DG)(DG)(DA) (DC)(DT)(DG)(DT)(DA)(DT)(DT)(DG)(DA)(DT) (DC) (DA)(DG)(DT)(DC)(DC)(DA) ...String: (DA)(DT)(DC)(DT)(DT)(DT)(DC)(DC)(DT)(DA) (DT)(DT)(DG)(DC)(DT)(DT)(DT)(DA)(DA)(DA) (DG)(DG)(DC)(DA)(DG)(DA)(DG)(DG)(DA) (DC)(DT)(DG)(DT)(DA)(DT)(DT)(DG)(DA)(DT) (DC) (DA)(DG)(DT)(DC)(DC)(DA)(DA)(DA) (DC)(DT)(DT)(DC)(DT)(DT)(DT)(DC)(DT)(DG) (DC)(DA) (DT)(DG)(DT)(DA)(DC)(DA)(DT) (DG)(DG)(DA)(DA)(DA)(DA)(DC)(DT)(DG)(DG) (DC)(DC)(DA) (DA)(DG)(DG)(DC)(DA)(DA) (DA)(DC)(DA)(DC)(DG)(DT)(DC)(DC)(DG)(DG) (DA)(DA)(DT)(DG) (DA)(DT)(DG)(DG)(DT) (DA)(DT)(DT)(DT)(DA)(DA)(DG)(DA)(DA)(DC) (DA)(DA)(DA)(DC)(DA) (DT)(DT)(DC)(DC) (DC)(DT)(DG)(DG)(DT)(DA)(DT)(DC)(DA)(DG) (DC)(DA)(DA)(DG)(DT)(DA) (DC)(DA)(DG) (DT)(DG)(DC)(DC)(DC)(DT)(DG)(DC)(DT)(DG) (DA)(DC)(DA)(DG)(DA)(DG)(DC) (DA)(DG) (DG)(DA)(DG)(DA)(DC)(DA)(DC)(DA)(DA)(DA) (DG)(DT)(DA)(DC)(DC)(DA)(DT)(DC) (DT) (DC)(DG)(DG)(DA)(DT) |
-Macromolecule #3: Histone H3.1
| Macromolecule | Name: Histone H3.1 / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.437167 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MARTKQTARK STGGKAPRKQ LATKAARKSA PATGGVKKPH RYRPGTVALR EIRRYQKSTE LLIRKLPFQR LVREIAQDFK TDLRFQSSA VMALQEACEA YLVGLFEDTN LCAIHAKRVT IMPKDIQLAR RIRGERA UniProtKB: Histone H3.1 |
-Macromolecule #4: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.394426 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKGGKG LGKGGAKRHR KVLRDNIQGI TKPAIRRLAR RGGVKRISGL IYEETRGVLK VFLENVIRDA VTYTEHAKRK TVTAMDVVY ALKRQGRTLY GFGG UniProtKB: Histone H4 |
-Macromolecule #5: Histone H2A type 1-B/E
| Macromolecule | Name: Histone H2A type 1-B/E / type: protein_or_peptide / ID: 5 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 14.165551 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKQGGK ARAKAKTRSS RAGLQFPVGR VHRLLRKGNY SERVGAGAPV YLAAVLEYLT AEILELAGNA ARDNKKTRII PRHLQLAIR NDEELNKLLG RVTIAQGGVL PNIQAVLLPK KTESHHKAKG K UniProtKB: Histone H2A type 1-B/E |
-Macromolecule #6: Histone H2B type 1-J
| Macromolecule | Name: Histone H2B type 1-J / type: protein_or_peptide / ID: 6 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.935239 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPEPAKSAPA PKKGSKKAVT KAQKKDGKKR KRSRKESYSI YVYKVLKQVH PDTGISSKAM GIMNSFVNDI FERIAGEASR LAHYNKRST ITSREIQTAV RLLLPGELAK HAVSEGTKAV TKYTSAK UniProtKB: Histone H2B type 1-J |
-Macromolecule #7: Maltose/maltodextrin-binding periplasmic protein,Transcription fa...
| Macromolecule | Name: Maltose/maltodextrin-binding periplasmic protein,Transcription factor GATA-4 type: protein_or_peptide / ID: 7 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 87.880258 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH GSSMKIEEGK LVIWINGDKG YNGLAEVGKK FEKDTGIKVT VEHPDKLEEK FPQVAATGDG PDIIFWAHDR FGGYAQSGL LAEITPDKAF QDKLYPFTWD AVRYNGKLIA YPIAVEALSL IYNKDLLPNP PKTWEEIPAL DKELKAKGKS A LMFNLQEP ...String: MGSSHHHHHH GSSMKIEEGK LVIWINGDKG YNGLAEVGKK FEKDTGIKVT VEHPDKLEEK FPQVAATGDG PDIIFWAHDR FGGYAQSGL LAEITPDKAF QDKLYPFTWD AVRYNGKLIA YPIAVEALSL IYNKDLLPNP PKTWEEIPAL DKELKAKGKS A LMFNLQEP YFTWPLIAAD GGYAFKYENG KYDIKDVGVD NAGAKAGLTF LVDLIKNKHM NADTDYSIAE AAFNKGETAM TI NGPWAWS NIDTSKVNYG VTVLPTFKGQ PSKPFVGVLS AGINAASPNK ELAKEFLENY LLTDEGLEAV NKDKPLGAVA LKS YEEELA KDPRIAATME NAQKGEIMPN IPQMSAFWYA VRTAVINAAS GRQTVDEALK DAQTNGIEEN LYFQSNAMYQ SLAM AANHG PPPGAYEAGG PGAFMHGAGA ASSPVYVPTP RVPSSVLGLS YLQGGGAGSA SGGASGGSSG GAASGAGPGT QQGSP GWSQ AGADGAAYTP PPVSPRFSFP GTTGSLAAAA AAAAAREAAA YSSGGGAAGA GLAGREQYGR AGFAGSYSSP YPAYMA DVG ASWAAAAAAS AGPFDSPVLH SLPGRANPAA RHPNLDMFDD FSEGRECVNC GAMSTPLWRR DGTGHYLCNA CGLYHKM NG INRPLIKPQR RLSASRRVGL SCANCQTTTT TLWRRNAEGE PVCNACGLYM KLHGVPRPLA MRKEGIQTRK RKPKNLNK S KTPAAPSGSE SLPPASGASS NSSNATTSSS EEMRPIKTEP GLSSHYGHSS SVSQTFSVSA MSGHGPSIHP VLSALKLSP QGYASPVSQS PQTSSKQDSW NSLVLADSHG DIITA UniProtKB: Maltose/maltodextrin-binding periplasmic protein, Transcription factor GATA-4 |
-Macromolecule #8: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 8 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 1.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)