[English] 日本語
 Yorodumi
Yorodumi- EMDB-43197: Cryo-EM structure of FoxA1 and GATA4 in complex with ALBN1 nucleosome -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of FoxA1 and GATA4 in complex with ALBN1 nucleosome | |||||||||
 Map data Map data | main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | nucleosome / pioneer transcription factors / DNA binding proteins / transcription / chromatin / NUCLEAR PROTEIN / NUCLEAR PROTEIN-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationalveolar secondary septum development / respiratory basal cell differentiation / positive regulation of dopaminergic neuron differentiation / atrioventricular valve formation / atrial septum secundum morphogenesis / embryonic heart tube anterior/posterior pattern specification / transdifferentiation / mesenchymal-epithelial cell signaling involved in prostate gland development / Formation of lateral plate mesoderm / anatomical structure formation involved in morphogenesis ...alveolar secondary septum development / respiratory basal cell differentiation / positive regulation of dopaminergic neuron differentiation / atrioventricular valve formation / atrial septum secundum morphogenesis / embryonic heart tube anterior/posterior pattern specification / transdifferentiation / mesenchymal-epithelial cell signaling involved in prostate gland development / Formation of lateral plate mesoderm / anatomical structure formation involved in morphogenesis / intestinal epithelial cell differentiation / prostate gland stromal morphogenesis / cardiac right ventricle morphogenesis / atrioventricular node development / positive regulation of cell-cell adhesion mediated by cadherin / co-SMAD binding / atrial septum morphogenesis / cardiac muscle tissue regeneration / cell growth involved in cardiac muscle cell development / epithelial cell maturation involved in prostate gland development / Transcriptional regulation of testis differentiation / cardiac ventricle morphogenesis / endocardial cushion development / neuron fate specification / Physiological factors / atrial septum primum morphogenesis / atrioventricular canal development / lung epithelial cell differentiation / embryonic foregut morphogenesis / secretory columnal luminar epithelial cell differentiation involved in prostate glandular acinus development / YAP1- and WWTR1 (TAZ)-stimulated gene expression / dorsal/ventral neural tube patterning / Synthesis, secretion, and inactivation of Glucose-dependent Insulinotropic Polypeptide (GIP) / prostate gland epithelium morphogenesis / Formation of definitive endoderm / endoderm development / Formation of axial mesoderm / dopaminergic neuron differentiation / positive regulation of BMP signaling pathway / Developmental Lineage of Pancreatic Acinar Cells / positive regulation of smoothened signaling pathway / hormone metabolic process / response to vitamin A / regulation of cardiac muscle cell contraction / Cardiogenesis / aortic valve morphogenesis / negative regulation of cardiac muscle cell apoptotic process / negative regulation of epithelial to mesenchymal transition / ventricular septum development / smoothened signaling pathway / positive regulation of intracellular estrogen receptor signaling pathway / DNA-binding transcription activator activity / NFAT protein binding / heart looping / detection of maltose stimulus / epithelial tube branching involved in lung morphogenesis / maltose transport complex / microvillus / carbohydrate transport / positive regulation of vascular endothelial growth factor production / negative regulation of apoptotic signaling pathway / carbohydrate transmembrane transporter activity / maltose binding / cell fate commitment / anatomical structure morphogenesis / maltose transport / maltodextrin transmembrane transport / negative regulation of tumor necrosis factor-mediated signaling pathway / response to mechanical stimulus / negative regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / : / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / Notch signaling pathway / CENP-A containing nucleosome / Packaging Of Telomere Ends / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / positive regulation of mitotic cell cycle / ATP-binding cassette (ABC) transporter complex / telomere organization / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Interleukin-7 signaling / negative regulation of autophagy / RNA Polymerase I Promoter Opening / epigenetic regulation of gene expression / Inhibition of DNA recombination at telomere / Assembly of the ORC complex at the origin of replication / Meiotic synapsis / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
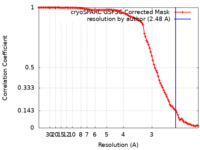
| Method | single particle reconstruction / cryo EM / Resolution: 2.48 Å | |||||||||
 Authors Authors | Zhou BR / Bai Y | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2024 Journal: Mol Cell / Year: 2024Title: Structural insights into the cooperative nucleosome recognition and chromatin opening by FOXA1 and GATA4. Authors: Bing-Rui Zhou / Hanqiao Feng / Furong Huang / Iris Zhu / Stephanie Portillo-Ledesma / Dan Shi / Kenneth S Zaret / Tamar Schlick / David Landsman / Qianben Wang / Yawen Bai /   Abstract: Mouse FOXA1 and GATA4 are prototypes of pioneer factors, initiating liver cell development by binding to the N1 nucleosome in the enhancer of the ALB1 gene. Using cryoelectron microscopy (cryo-EM), ...Mouse FOXA1 and GATA4 are prototypes of pioneer factors, initiating liver cell development by binding to the N1 nucleosome in the enhancer of the ALB1 gene. Using cryoelectron microscopy (cryo-EM), we determined the structures of the free N1 nucleosome and its complexes with FOXA1 and GATA4, both individually and in combination. We found that the DNA-binding domains of FOXA1 and GATA4 mainly recognize the linker DNA and an internal site in the nucleosome, respectively, whereas their intrinsically disordered regions interact with the acidic patch on histone H2A-H2B. FOXA1 efficiently enhances GATA4 binding by repositioning the N1 nucleosome. In vivo DNA editing and bioinformatics analyses suggest that the co-binding mode of FOXA1 and GATA4 plays important roles in regulating genes involved in liver cell functions. Our results reveal the mechanism whereby FOXA1 and GATA4 cooperatively bind to the nucleosome through nucleosome repositioning, opening chromatin by bending linker DNA and obstructing nucleosome packing. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43197.map.gz emd_43197.map.gz | 96.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43197-v30.xml emd-43197-v30.xml emd-43197.xml emd-43197.xml | 27.9 KB 27.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_43197_fsc.xml emd_43197_fsc.xml | 11.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_43197.png emd_43197.png | 109.5 KB | ||
| Filedesc metadata |  emd-43197.cif.gz emd-43197.cif.gz | 8.3 KB | ||
| Others |  emd_43197_half_map_1.map.gz emd_43197_half_map_1.map.gz emd_43197_half_map_2.map.gz emd_43197_half_map_2.map.gz | 3.7 MB 3.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43197 http://ftp.pdbj.org/pub/emdb/structures/EMD-43197 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43197 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43197 | HTTPS FTP |
-Validation report
| Summary document |  emd_43197_validation.pdf.gz emd_43197_validation.pdf.gz | 495.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_43197_full_validation.pdf.gz emd_43197_full_validation.pdf.gz | 494.8 KB | Display | |
| Data in XML |  emd_43197_validation.xml.gz emd_43197_validation.xml.gz | 17.9 KB | Display | |
| Data in CIF |  emd_43197_validation.cif.gz emd_43197_validation.cif.gz | 23.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43197 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43197 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43197 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43197 | HTTPS FTP |
-Related structure data
| Related structure data |  8vg1MC  8vfxC  8vfyC  8vfzC  8vg0C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_43197.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43197.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.056 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half B
| File | emd_43197_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half A
| File | emd_43197_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : FoxA1 and GATA4 in complx with 186bp ALBN1 nucleosome
+Supramolecule #1: FoxA1 and GATA4 in complx with 186bp ALBN1 nucleosome
+Macromolecule #1: Histone H3.1
+Macromolecule #2: Histone H4
+Macromolecule #3: Histone H2A type 1-B/E
+Macromolecule #4: Histone H2B type 1-J
+Macromolecule #7: Hepatocyte nuclear factor 3-alpha
+Macromolecule #8: Maltose/maltodextrin-binding periplasmic protein,Transcription fa...
+Macromolecule #5: DNA (171-MER)
+Macromolecule #6: DNA (171-MER)
+Macromolecule #9: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 1.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)