[English] 日本語
 Yorodumi
Yorodumi- EMDB-42509: Intracellular cryo-tomography structure of EBOV nucleocapsid at 8... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Intracellular cryo-tomography structure of EBOV nucleocapsid at 8.9 Angstrom | |||||||||
 Map data Map data | map sharp | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | VIRAL PROTEIN / nucleoprotein / nucleocapsid / Ebola virus / EBOV / filovirus / subtomogram averaging / cryo-ET / FIB / intracellular / in situ | |||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell endomembrane system / symbiont-mediated suppression of host defenses / symbiont-mediated suppression of host RNAi-mediated antiviral immune response / negative regulation of miRNA-mediated gene silencing / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of IKBKE activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of IRF7 activity / symbiont-mediated suppression of host antigen processing and presentation of peptide antigen via MHC class II / symbiont-mediated suppression of host PKR/eIFalpha signaling / positive regulation of protein sumoylation / viral RNA genome packaging ...host cell endomembrane system / symbiont-mediated suppression of host defenses / symbiont-mediated suppression of host RNAi-mediated antiviral immune response / negative regulation of miRNA-mediated gene silencing / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of IKBKE activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of IRF7 activity / symbiont-mediated suppression of host antigen processing and presentation of peptide antigen via MHC class II / symbiont-mediated suppression of host PKR/eIFalpha signaling / positive regulation of protein sumoylation / viral RNA genome packaging / helical viral capsid / viral transcription / molecular sequestering activity / symbiont-mediated suppression of host JAK-STAT cascade via inhibition of STAT1 activity / viral genome replication / viral budding from plasma membrane / viral nucleocapsid / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of TBK1 activity / symbiont-mediated suppression of host toll-like receptor signaling pathway / host cell cytoplasm / symbiont-mediated suppression of host innate immune response / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / ribonucleoprotein complex / negative regulation of gene expression / host cell plasma membrane / virion membrane / structural molecule activity / RNA binding / membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 8.9 Å | |||||||||
 Authors Authors | Watanabe R / Zyla D / Saphire EO | |||||||||
| Funding support | 1 items
| |||||||||
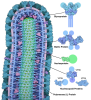
 Citation Citation |  Journal: Cell / Year: 2024 Journal: Cell / Year: 2024Title: Intracellular Ebola virus nucleocapsid assembly revealed by in situ cryo-electron tomography. Authors: Reika Watanabe / Dawid Zyla / Diptiben Parekh / Connor Hong / Ying Jones / Sharon L Schendel / William Wan / Guillaume Castillon / Erica Ollmann Saphire /  Abstract: Filoviruses, including the Ebola and Marburg viruses, cause hemorrhagic fevers with up to 90% lethality. The viral nucleocapsid is assembled by polymerization of the nucleoprotein (NP) along the ...Filoviruses, including the Ebola and Marburg viruses, cause hemorrhagic fevers with up to 90% lethality. The viral nucleocapsid is assembled by polymerization of the nucleoprotein (NP) along the viral genome, together with the viral proteins VP24 and VP35. We employed cryo-electron tomography of cells transfected with viral proteins and infected with model Ebola virus to illuminate assembly intermediates, as well as a 9 Å map of the complete intracellular assembly. This structure reveals a previously unresolved third and outer layer of NP complexed with VP35. The intrinsically disordered region, together with the C-terminal domain of this outer layer of NP, provides the constant width between intracellular nucleocapsid bundles and likely functions as a flexible tether to the viral matrix protein in the virion. A comparison of intracellular nucleocapsids with prior in-virion nucleocapsid structures reveals that the nucleocapsid further condenses vertically in the virion. The interfaces responsible for nucleocapsid assembly are highly conserved and offer targets for broadly effective antivirals. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42509.map.gz emd_42509.map.gz | 40 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42509-v30.xml emd-42509-v30.xml emd-42509.xml emd-42509.xml | 27.9 KB 27.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_42509.png emd_42509.png | 144.8 KB | ||
| Filedesc metadata |  emd-42509.cif.gz emd-42509.cif.gz | 8.1 KB | ||
| Others |  emd_42509_half_map_1.map.gz emd_42509_half_map_1.map.gz emd_42509_half_map_2.map.gz emd_42509_half_map_2.map.gz | 21.9 MB 21.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42509 http://ftp.pdbj.org/pub/emdb/structures/EMD-42509 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42509 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42509 | HTTPS FTP |
-Related structure data
| Related structure data |  8usnMC  8ustC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42509.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42509.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map sharp | ||||||||||||||||||||||||||||||||||||
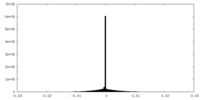
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.149 Å | ||||||||||||||||||||||||||||||||||||
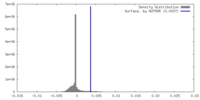
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half 2
| File | emd_42509_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
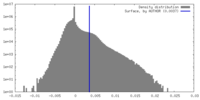
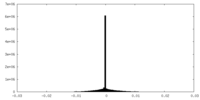
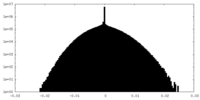
| Density Histograms |
-Half map: half 1
| File | emd_42509_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
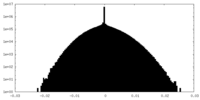
| Density Histograms |
- Sample components
Sample components
-Entire : Complex
| Entire | Name: Complex |
|---|---|
| Components |
|
-Supramolecule #1: Complex
| Supramolecule | Name: Complex / type: virus / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 Details: 3 virus proteins were expressed from a plasmid in HEK 293T cells Virus type: VIRUS-LIKE PARTICLE / Virus isolate: OTHER / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #2: NP core with RNA
| Supramolecule | Name: NP core with RNA / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: Outer NP with VP35 NP-binding peptide
| Supramolecule | Name: Outer NP with VP35 NP-binding peptide / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #1, #4 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #4: VP24 second layer of nucleocapsid
| Supramolecule | Name: VP24 second layer of nucleocapsid / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #5: VP35 CTD
| Supramolecule | Name: VP35 CTD / type: complex / ID: 5 / Parent: 1 / Macromolecule list: #4 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Nucleoprotein
| Macromolecule | Name: Nucleoprotein / type: protein_or_peptide / ID: 1 / Details: The N-terminal part of nucleoprotein / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 83.3875 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDSRPQKIWM APSLTESDMD YHKILTAGLS VQQGIVRQRV IPVYQVNNLE EICQLIIQAF EAGVDFQESA DSFLLMLCLH HAYQGDYKL FLESGAVKYL EGHGFRFEVK KRDGVKRLEE LLPAVSSGKN IKRTLAAMPE EETTEANAGQ FLSFASLFLP K LVVGEKAC ...String: MDSRPQKIWM APSLTESDMD YHKILTAGLS VQQGIVRQRV IPVYQVNNLE EICQLIIQAF EAGVDFQESA DSFLLMLCLH HAYQGDYKL FLESGAVKYL EGHGFRFEVK KRDGVKRLEE LLPAVSSGKN IKRTLAAMPE EETTEANAGQ FLSFASLFLP K LVVGEKAC LEKVQRQIQV HAEQGLIQYP TAWQSVGHMM VIFRLMRTNF LIKFLLIHQG MHMVAGHDAN DAVISNSVAQ AR FSGLLIV KTVLDHILQK TERGVRLHPL ARTAKVKNEV NSFKAALSSL AKHGEYAPFA RLLNLSGVNN LEHGLFPQLS AIA LGVATA HGSTLAGVNV GEQYQQLREA ATEAEKQLQQ YAESRELDHL GLDDQEKKIL MNFHQKKNEI SFQQTNAMVT LRKE RLAKL TEAITAASLP KTSGHYDDDD DIPFPGPIND DDNPGHQDDD PTDSQDTTIP DVVVDPDDGS YGEYQSYSEN GMNAP DDLV LFDLDEDDED TKPVPNRSTK GGQQKNSQKG QHIEGRQTQS RPIQNVPGPH RTIHHASAPL TDNDRRNEPS GSTSPR MLT PINEEADPLD DADDETSSLP PLESDDEEQD RDGTSNRTPT VAPPAPVYRD HSEKKELPQD EQQDQDHTQE ARNQDSD NT QSEHSFEEMY RHILRSQGPF DAVLYYHMMK DEPVVFSTSD GKEYTYPDSL EEEYPPWLTE KEAMNEENRF VTLDGQQF Y WPVMNHKNKF MAILQHHQ UniProtKB: Nucleoprotein |
-Macromolecule #3: Membrane-associated protein VP24
| Macromolecule | Name: Membrane-associated protein VP24 / type: protein_or_peptide / ID: 3 / Details: VP24 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 28.250811 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAKATGRYNL ISPKKDLEKG VVLSDLCNFL VSQTIQGWKV YWAGIEFDVT HKGMALLHRL KTNDFAPAWS MTRNLFPHLF QNPNSTIES PLWALRVILA AGIQDQLIDQ SLIEPLAGAL GLISDWLLTT NTNHFNMRTQ RVKEQLSLKM LSLIRSNILK F INKLDALH ...String: MAKATGRYNL ISPKKDLEKG VVLSDLCNFL VSQTIQGWKV YWAGIEFDVT HKGMALLHRL KTNDFAPAWS MTRNLFPHLF QNPNSTIES PLWALRVILA AGIQDQLIDQ SLIEPLAGAL GLISDWLLTT NTNHFNMRTQ RVKEQLSLKM LSLIRSNILK F INKLDALH VVNYNGLLSS IEIGTQNHTI IITRTNMGFL VELQEPDKSA MNRMKPGPAK FSLLHESTLK AFTQGSSTRM QS LILEFNS SLAI UniProtKB: Membrane-associated protein VP24 |
-Macromolecule #4: Polymerase cofactor VP35
| Macromolecule | Name: Polymerase cofactor VP35 / type: protein_or_peptide / ID: 4 / Details: C-terminal domain of VP35 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 37.403277 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MTTRTKGRGH TAATTQNDRM PGPELSGWIS EQLMTGRIPV SDIFCDIENN PGLCYASQMQ QTKPNPKTRN SQTQTDPICN HSFEEVVQT LASLATVVQQ QTIASESLEQ RITSLENGLK PVYDMAKTIS SLNRVCAEMV AKYDLLVMTT GRATATAAAT E AYWAEHGQ ...String: MTTRTKGRGH TAATTQNDRM PGPELSGWIS EQLMTGRIPV SDIFCDIENN PGLCYASQMQ QTKPNPKTRN SQTQTDPICN HSFEEVVQT LASLATVVQQ QTIASESLEQ RITSLENGLK PVYDMAKTIS SLNRVCAEMV AKYDLLVMTT GRATATAAAT E AYWAEHGQ PPPGPSLYEE SAIRGKIESR DETVPQSVRE AFNNLNSTTS LTEENFGKPD ISAKDLRNIM YDHLPGFGTA FH QLVQVIC KLGKDSNSLD IIHAEFQASL AEGDSPQCAL IQITKRVPIF QDAAPPVIHI RSRGDIPRAC QKSLRPVPPS PKI DRGWVC VFQLQDGKTL GLKI UniProtKB: Polymerase cofactor VP35 |
-Macromolecule #2: RNA (5'-R(*AP*AP*AP*AP*AP*A)-3')
| Macromolecule | Name: RNA (5'-R(*AP*AP*AP*AP*AP*A)-3') / type: rna / ID: 2 / Details: Sample RNA sequence / Number of copies: 2 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 1.930277 KDa |
| Sequence | String: AAAAAA |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.2 |
|---|---|
| Grid | Model: Quantifoil / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE-PROPANE |
| Details | FIB-milled-plunge-frozen cell expressing EBOV NP(601-739 truncated), VP24 and VP35 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 3.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 2.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-8usn: |
 Movie
Movie Controller
Controller



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)