[English] 日本語
 Yorodumi
Yorodumi- EMDB-41506: Human parainfluenza virus type 3 prefusion F trimer in complex wi... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human parainfluenza virus type 3 prefusion F trimer in complex with rPIV3-18 Fab | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Immune system / viral neutralization / VIRAL PROTEIN | |||||||||
| Function / homology | Precursor fusion glycoprotein F0, Paramyxoviridae / Fusion glycoprotein F0 / fusion of virus membrane with host plasma membrane / viral envelope / symbiont entry into host cell / host cell plasma membrane / virion membrane / Fusion glycoprotein F0 Function and homology information Function and homology information | |||||||||
| Biological species |  Human respirovirus 3 / Human respirovirus 3 /  Homo sapiens (human) Homo sapiens (human) | |||||||||
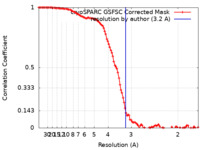
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Otrelo-Cardoso AR / Jardetzky TS | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2024 Journal: Nat Microbiol / Year: 2024Title: Functional and structural basis of human parainfluenza virus type 3 neutralization with human monoclonal antibodies. Authors: Naveenchandra Suryadevara / Ana Rita Otrelo-Cardoso / Nurgun Kose / Yao-Xiong Hu / Elad Binshtein / Rachael M Wolters / Alexander L Greninger / Laura S Handal / Robert H Carnahan / Anne ...Authors: Naveenchandra Suryadevara / Ana Rita Otrelo-Cardoso / Nurgun Kose / Yao-Xiong Hu / Elad Binshtein / Rachael M Wolters / Alexander L Greninger / Laura S Handal / Robert H Carnahan / Anne Moscona / Theodore S Jardetzky / James E Crowe /  Abstract: Human parainfluenza virus type 3 (hPIV3) is a respiratory pathogen that can cause severe disease in older people and infants. Currently, vaccines against hPIV3 are in clinical trials but none have ...Human parainfluenza virus type 3 (hPIV3) is a respiratory pathogen that can cause severe disease in older people and infants. Currently, vaccines against hPIV3 are in clinical trials but none have been approved yet. The haemagglutinin-neuraminidase (HN) and fusion (F) surface glycoproteins of hPIV3 are major antigenic determinants. Here we describe naturally occurring potently neutralizing human antibodies directed against both surface glycoproteins of hPIV3. We isolated seven neutralizing HN-reactive antibodies and a pre-fusion conformation F-reactive antibody from human memory B cells. One HN-binding monoclonal antibody (mAb), designated PIV3-23, exhibited functional attributes including haemagglutination and neuraminidase inhibition. We also delineated the structural basis of neutralization for two HN and one F mAbs. MAbs that neutralized hPIV3 in vitro protected against infection and disease in vivo in a cotton rat model of hPIV3 infection, suggesting correlates of protection for hPIV3 and the potential clinical utility of these mAbs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41506.map.gz emd_41506.map.gz | 62.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41506-v30.xml emd-41506-v30.xml emd-41506.xml emd-41506.xml | 21.9 KB 21.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41506_fsc.xml emd_41506_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_41506.png emd_41506.png | 430.5 KB | ||
| Filedesc metadata |  emd-41506.cif.gz emd-41506.cif.gz | 7 KB | ||
| Others |  emd_41506_half_map_1.map.gz emd_41506_half_map_1.map.gz emd_41506_half_map_2.map.gz emd_41506_half_map_2.map.gz | 115.9 MB 115.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41506 http://ftp.pdbj.org/pub/emdb/structures/EMD-41506 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41506 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41506 | HTTPS FTP |
-Related structure data
| Related structure data |  8tqkMC  8tqiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41506.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41506.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_41506_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
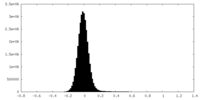
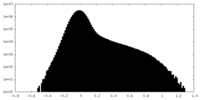
| Density Histograms |
-Half map: #1
| File | emd_41506_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
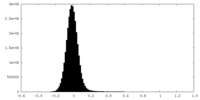
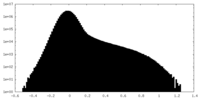
| Density Histograms |
- Sample components
Sample components
-Entire : HPIV3 prefusion F trimer in complex with rPIV3-18 Fab
| Entire | Name: HPIV3 prefusion F trimer in complex with rPIV3-18 Fab |
|---|---|
| Components |
|
-Supramolecule #1: HPIV3 prefusion F trimer in complex with rPIV3-18 Fab
| Supramolecule | Name: HPIV3 prefusion F trimer in complex with rPIV3-18 Fab / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Human respirovirus 3 Human respirovirus 3 |
-Supramolecule #2: Fusion glycoprotein F0
| Supramolecule | Name: Fusion glycoprotein F0 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  Human respirovirus 3 Human respirovirus 3 |
-Supramolecule #3: rPIV3-18 light chain
| Supramolecule | Name: rPIV3-18 light chain / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #4: rPIV3-18 heavy chain
| Supramolecule | Name: rPIV3-18 heavy chain / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Heavy chain Fab rPIV3-18
| Macromolecule | Name: Heavy chain Fab rPIV3-18 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.959973 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: EVQLLESGGG LVRPGGSLRL SCAASGFTFS KYVMAWVRQA PGKGLEWVSS ITSGERTFYA DSVKGRFTVS RDNSKYTLDL EMSRLRAED TAVYYCAKMF KWDYDTSDFG VWGQGTLVTV SSASTKGPSV FPLAPSSKST SGGTAALGCL VKDYFPEPVT V SWNSGALT ...String: EVQLLESGGG LVRPGGSLRL SCAASGFTFS KYVMAWVRQA PGKGLEWVSS ITSGERTFYA DSVKGRFTVS RDNSKYTLDL EMSRLRAED TAVYYCAKMF KWDYDTSDFG VWGQGTLVTV SSASTKGPSV FPLAPSSKST SGGTAALGCL VKDYFPEPVT V SWNSGALT SGVHTFPAVL QSSGLYSLSS VVTVPSSSLG TQTYICNVNH KPSNTKVDKK VEPKSC |
-Macromolecule #2: Light chain Fab rPIV3-28
| Macromolecule | Name: Light chain Fab rPIV3-28 / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.671273 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DFQMTQSPST LSASVGDRVT ITCRASQSVG NWLTWYQHKP GKAPKILIYK ASTLQSGVPS RFSGSGSGTE FTLTISSLQP DDFATYYCQ QFNTYSWTFG QGTRVEIKRT VAAPSVFIFP PSDEQLKSGT ASVVCLLNNF YPREAKVQWK VDNALQSGNS Q ESVTEQDS ...String: DFQMTQSPST LSASVGDRVT ITCRASQSVG NWLTWYQHKP GKAPKILIYK ASTLQSGVPS RFSGSGSGTE FTLTISSLQP DDFATYYCQ QFNTYSWTFG QGTRVEIKRT VAAPSVFIFP PSDEQLKSGT ASVVCLLNNF YPREAKVQWK VDNALQSGNS Q ESVTEQDS KDSTYSLSST LTLSKADYEK HKVYACEVTH QGLRSPVTKS FNRGEC |
-Macromolecule #3: Fusion glycoprotein F0
| Macromolecule | Name: Fusion glycoprotein F0 / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human respirovirus 3 Human respirovirus 3 |
| Molecular weight | Theoretical: 57.360102 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QIDITKLQHV GVLVNSPKGM KISQNFETRY LILSLIPKIE DSNSCGDQQI KQYKRLLDRL IIPLYDGLKL QKDVIVTNQE SNENTDPRT ERFFGGVIGT IALGVATSAQ ITAAVALVEA KQAKSDIEKL KEAIRDTNKA VQSVCSSVGN CIVAIKSVQD Y VNKEIVPS ...String: QIDITKLQHV GVLVNSPKGM KISQNFETRY LILSLIPKIE DSNSCGDQQI KQYKRLLDRL IIPLYDGLKL QKDVIVTNQE SNENTDPRT ERFFGGVIGT IALGVATSAQ ITAAVALVEA KQAKSDIEKL KEAIRDTNKA VQSVCSSVGN CIVAIKSVQD Y VNKEIVPS IARLGCEAAG LQLGIALTQH YSELTNCFGD NIGSLQEKGI KLQCIASLYR TNITEIFTTS TVDKYDIYDL LF TESIKVR VIDVDLNDYS ITLQVRLPLL TRLLNTQIYK VDSISYNIQN REWYIPLPSH IMTKGAFLGG ADVKECIEAF SSY ICPSDP GFVLNHEMES CLSGNISQCP RTTVTSDIVP RYAFVNGGVV ANCITTTCTC NGIGNRINQP PDQGVKIITH KECN TIGIN GMLFNTNKEG TLAFYTPDDI TLNNSVALDP IDISIELNKV KSDLEESKEW YRRSNQKLSA IEDKIEEILS KIYHI ENEI ARIKKLIGEA PGSENLYFQG GSGSHHHHHH HH UniProtKB: Fusion glycoprotein F0 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.15 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)