+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Acinetobacter GP16 Type IV pilus | |||||||||
 Map data Map data | map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | T4P / Competence / CELL ADHESION | |||||||||
| Function / homology |  Function and homology information Function and homology informationprotein secretion by the type II secretion system / type II protein secretion system complex / pilus / cell adhesion / membrane Similarity search - Function | |||||||||
| Biological species |  Acinetobacter genomosp. 16BJ (bacteria) Acinetobacter genomosp. 16BJ (bacteria) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.14 Å | |||||||||
 Authors Authors | Meng R / Xing Z / Zhang J | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural basis of Acinetobacter type IV pili targeting by an RNA virus. Authors: Ran Meng / Zhongliang Xing / Jeng-Yih Chang / Zihao Yu / Jirapat Thongchol / Wen Xiao / Yuhang Wang / Karthik Chamakura / Zhiqi Zeng / Fengbin Wang / Ry Young / Lanying Zeng / Junjie Zhang /  Abstract: Acinetobacters pose a significant threat to human health, especially those with weakened immune systems. Type IV pili of acinetobacters play crucial roles in virulence and antibiotic resistance. ...Acinetobacters pose a significant threat to human health, especially those with weakened immune systems. Type IV pili of acinetobacters play crucial roles in virulence and antibiotic resistance. Single-stranded RNA bacteriophages target the bacterial retractile pili, including type IV. Our study delves into the interaction between Acinetobacter phage AP205 and type IV pili. Using cryo-electron microscopy, we solve structures of the AP205 virion with an asymmetric dimer of maturation proteins, the native Acinetobacter type IV pili bearing a distinct post-translational pilin cleavage, and the pili-bound AP205 showing its maturation proteins adapted to pilin modifications, allowing each phage to bind to one or two pili. Leveraging these results, we develop a 20-kilodalton AP205-derived protein scaffold targeting type IV pili in situ, with potential for research and diagnostics. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41442.map.gz emd_41442.map.gz | 122.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41442-v30.xml emd-41442-v30.xml emd-41442.xml emd-41442.xml | 17.5 KB 17.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41442_fsc.xml emd_41442_fsc.xml | 16.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_41442.png emd_41442.png | 51.1 KB | ||
| Filedesc metadata |  emd-41442.cif.gz emd-41442.cif.gz | 5.5 KB | ||
| Others |  emd_41442_half_map_1.map.gz emd_41442_half_map_1.map.gz emd_41442_half_map_2.map.gz emd_41442_half_map_2.map.gz | 226.3 MB 226.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41442 http://ftp.pdbj.org/pub/emdb/structures/EMD-41442 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41442 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41442 | HTTPS FTP |
-Validation report
| Summary document |  emd_41442_validation.pdf.gz emd_41442_validation.pdf.gz | 941 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41442_full_validation.pdf.gz emd_41442_full_validation.pdf.gz | 940.6 KB | Display | |
| Data in XML |  emd_41442_validation.xml.gz emd_41442_validation.xml.gz | 21.2 KB | Display | |
| Data in CIF |  emd_41442_validation.cif.gz emd_41442_validation.cif.gz | 27.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41442 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41442 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41442 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41442 | HTTPS FTP |
-Related structure data
| Related structure data |  8tobMC  8tocC  8tv9C  8tvaC  8tw2C  8twcC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41442.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41442.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.832 Å | ||||||||||||||||||||||||||||||||||||
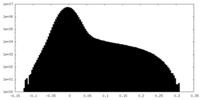
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: A
| File | emd_41442_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A | ||||||||||||
| Projections & Slices |
| ||||||||||||
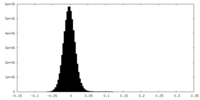
| Density Histograms |
-Half map: B
| File | emd_41442_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B | ||||||||||||
| Projections & Slices |
| ||||||||||||
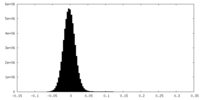
| Density Histograms |
- Sample components
Sample components
-Entire : Acinetobacter genomosp.16 Tyoe IV pilus
| Entire | Name: Acinetobacter genomosp.16 Tyoe IV pilus |
|---|---|
| Components |
|
-Supramolecule #1: Acinetobacter genomosp.16 Tyoe IV pilus
| Supramolecule | Name: Acinetobacter genomosp.16 Tyoe IV pilus / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Filamentous Type IV pilus |
|---|---|
| Source (natural) | Organism:  Acinetobacter genomosp. 16BJ (bacteria) Acinetobacter genomosp. 16BJ (bacteria) |
| Molecular weight | Theoretical: 11 kDa/nm |
-Macromolecule #1: Fimbrial protein
| Macromolecule | Name: Fimbrial protein / type: protein_or_peptide / ID: 1 / Number of copies: 22 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Acinetobacter genomosp. 16BJ (bacteria) Acinetobacter genomosp. 16BJ (bacteria) |
| Molecular weight | Theoretical: 7.470747 KDa |
| Sequence | String: TLIELMIVVA IIGILAAIAI PQYQNYIAKS QVSRVMSETG SLKTVIETCI LDGKTAANCE LGWTNSNLLG UniProtKB: Fimbrial protein |
-Macromolecule #2: Fimbrial protein
| Macromolecule | Name: Fimbrial protein / type: protein_or_peptide / ID: 2 / Number of copies: 22 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Acinetobacter genomosp. 16BJ (bacteria) Acinetobacter genomosp. 16BJ (bacteria) |
| Molecular weight | Theoretical: 6.999778 KDa |
| Sequence | String: STAAVTGQTG LTITYPASAT ESAAIQGTFG NSAAIKIKNQ TLTWTRTPEG AWSCATTVEA KFKPAGCAS UniProtKB: Fimbrial protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 1.35 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: 20mM Tris-HCl, 200mM NaCl, pH 8.0 | |||||||||
| Grid | Model: EMS Lacey Carbon / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 100 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK III / Details: 3uL sample applied to a QuantiFoil R2/1 grid. | |||||||||
| Details | vitreous Typy IV pilus |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.2 µm / Nominal magnification: 135000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: BACKBONE TRACE |
|---|---|
| Output model |  PDB-8tob: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)