+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of tail tube protein | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | cryo-EM structure of a protein / ANTIVIRAL PROTEIN | |||||||||
| Biological species |  | |||||||||
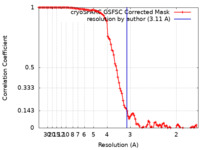
| Method | single particle reconstruction / cryo EM / Resolution: 3.11 Å | |||||||||
 Authors Authors | Zhang H / Li Z / Li XZ | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Insights into the modulation of bacterial NADase activity by phage proteins. Authors: Hang Yin / Xuzichao Li / Xiaoshen Wang / Chendi Zhang / Jiaqi Gao / Guimei Yu / Qiuqiu He / Jie Yang / Xiang Liu / Yong Wei / Zhuang Li / Heng Zhang /  Abstract: The Silent Information Regulator 2 (SIR2) protein is widely implicated in antiviral response by depleting the cellular metabolite NAD. The defense-associated sirtuin 2 (DSR2) effector, a SIR2 domain- ...The Silent Information Regulator 2 (SIR2) protein is widely implicated in antiviral response by depleting the cellular metabolite NAD. The defense-associated sirtuin 2 (DSR2) effector, a SIR2 domain-containing protein, protects bacteria from phage infection by depleting NAD, while an anti-DSR2 protein (DSR anti-defense 1, DSAD1) is employed by some phages to evade this host defense. The NADase activity of DSR2 is unleashed by recognizing the phage tail tube protein (TTP). However, the activation and inhibition mechanisms of DSR2 are unclear. Here, we determine the cryo-EM structures of DSR2 in multiple states. DSR2 is arranged as a dimer of dimers, which is facilitated by the tetramerization of SIR2 domains. Moreover, the DSR2 assembly is essential for activating the NADase function. The activator TTP binding would trigger the opening of the catalytic pocket and the decoupling of the N-terminal SIR2 domain from the C-terminal domain (CTD) of DSR2. Importantly, we further show that the activation mechanism is conserved among other SIR2-dependent anti-phage systems. Interestingly, the inhibitor DSAD1 mimics TTP to trap DSR2, thus occupying the TTP-binding pocket and inhibiting the NADase function. Together, our results provide molecular insights into the regulatory mechanism of SIR2-dependent NAD depletion in antiviral immunity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_38421.map.gz emd_38421.map.gz | 117.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-38421-v30.xml emd-38421-v30.xml emd-38421.xml emd-38421.xml | 15.7 KB 15.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_38421_fsc.xml emd_38421_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_38421.png emd_38421.png | 63.9 KB | ||
| Filedesc metadata |  emd-38421.cif.gz emd-38421.cif.gz | 5.7 KB | ||
| Others |  emd_38421_half_map_1.map.gz emd_38421_half_map_1.map.gz emd_38421_half_map_2.map.gz emd_38421_half_map_2.map.gz | 115.7 MB 115.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-38421 http://ftp.pdbj.org/pub/emdb/structures/EMD-38421 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38421 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-38421 | HTTPS FTP |
-Related structure data
| Related structure data |  8xknMC  8k98C  8k9aC  8w56C  8wknC M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_38421.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_38421.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_38421_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_38421_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of tail tube protein
| Entire | Name: Cryo-EM structure of tail tube protein |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of tail tube protein
| Supramolecule | Name: Cryo-EM structure of tail tube protein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: a protein
| Macromolecule | Name: a protein / type: protein_or_peptide / ID: 1 / Number of copies: 24 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 29.304701 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTVIQDTAD VYFKRKSDGK LVFTAEAQTA SFSQAISEEK LRGGIGNKPL YILKSEKEIN LTVKNAFFDL EWLAMTQGET IQEETKVKV FDREHGLIVD DTNKVTLKGK PVSDVTFYNK KGLTYKIAVS TDGTYTIPTA FAAAKDKLTA VYQIEKVGRR L AIKASKFS ...String: MKTVIQDTAD VYFKRKSDGK LVFTAEAQTA SFSQAISEEK LRGGIGNKPL YILKSEKEIN LTVKNAFFDL EWLAMTQGET IQEETKVKV FDREHGLIVD DTNKVTLKGK PVSDVTFYNK KGLTYKIAVS TDGTYTIPTA FAAAKDKLTA VYQIEKVGRR L AIKASKFS ERYEVEYRTI AYNPDTEEVY SDIYIQFPNV SPSGEFEMSL ENGNALAPEI KFEALADTDT DEMAVVIEAS RD ENTAAPV EDTTGSTQSS DLGGTTE |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)