+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | C-reactive protein, decamer | |||||||||
 Map data Map data | Relion sharpened full map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Pentraxin / Immune System / Acute-phase reactant | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of interleukin-8 production / opsonization / complement component C1q complex binding / low-density lipoprotein particle binding / vasoconstriction / choline binding / negative regulation of mononuclear cell proliferation / Classical antibody-mediated complement activation / low-density lipoprotein particle receptor binding / negative regulation of macrophage derived foam cell differentiation ...regulation of interleukin-8 production / opsonization / complement component C1q complex binding / low-density lipoprotein particle binding / vasoconstriction / choline binding / negative regulation of mononuclear cell proliferation / Classical antibody-mediated complement activation / low-density lipoprotein particle receptor binding / negative regulation of macrophage derived foam cell differentiation / negative regulation of lipid storage / positive regulation of superoxide anion generation / acute-phase response / defense response to Gram-positive bacterium / inflammatory response / innate immune response / calcium ion binding / positive regulation of gene expression / extracellular space / extracellular region / identical protein binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Yadav S / Vinothkumar KR | |||||||||
| Funding support |  India, 2 items India, 2 items
| |||||||||
 Citation Citation |  Journal: Acta Crystallogr D Struct Biol / Year: 2024 Journal: Acta Crystallogr D Struct Biol / Year: 2024Title: Factors affecting macromolecule orientations in thin films formed in cryo-EM. Authors: Swati Yadav / Kutti R Vinothkumar /  Abstract: The formation of a vitrified thin film embedded with randomly oriented macromolecules is an essential prerequisite for cryogenic sample electron microscopy. Most commonly, this is achieved using the ...The formation of a vitrified thin film embedded with randomly oriented macromolecules is an essential prerequisite for cryogenic sample electron microscopy. Most commonly, this is achieved using the plunge-freeze method first described nearly 40 years ago. Although this is a robust method, the behaviour of different macromolecules shows great variation upon freezing and often needs to be optimized to obtain an isotropic, high-resolution reconstruction. For a macromolecule in such a film, the probability of encountering the air-water interface in the time between blotting and freezing and adopting preferred orientations is very high. 3D reconstruction using preferentially oriented particles often leads to anisotropic and uninterpretable maps. Currently, there are no general solutions to this prevalent issue, but several approaches largely focusing on sample preparation with the use of additives and novel grid modifications have been attempted. In this study, the effect of physical and chemical factors on the orientations of macromolecules was investigated through an analysis of selected well studied macromolecules, and important parameters that determine the behaviour of proteins on cryo-EM grids were revealed. These insights highlight the nature of the interactions that cause preferred orientations and can be utilized to systematically address orientation bias for any given macromolecule and to provide a framework to design small-molecule additives to enhance sample stability and behaviour. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37865.map.gz emd_37865.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37865-v30.xml emd-37865-v30.xml emd-37865.xml emd-37865.xml | 17.3 KB 17.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_37865_fsc.xml emd_37865_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_37865.png emd_37865.png | 18.7 KB | ||
| Filedesc metadata |  emd-37865.cif.gz emd-37865.cif.gz | 5.9 KB | ||
| Others |  emd_37865_half_map_1.map.gz emd_37865_half_map_1.map.gz emd_37865_half_map_2.map.gz emd_37865_half_map_2.map.gz | 49.7 MB 49.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37865 http://ftp.pdbj.org/pub/emdb/structures/EMD-37865 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37865 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37865 | HTTPS FTP |
-Related structure data
| Related structure data |  8wv5MC  8wv4C  8wv6C  8wzhC  8wziC  8wzjC  8wzkC  8wzmC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_37865.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37865.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Relion sharpened full map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
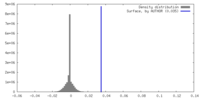
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_37865_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
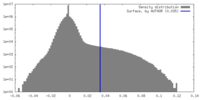
| Density Histograms |
-Half map: #1
| File | emd_37865_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
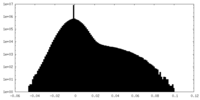
| Density Histograms |
- Sample components
Sample components
-Entire : C-reactive protein purified from human serum with calcium
| Entire | Name: C-reactive protein purified from human serum with calcium |
|---|---|
| Components |
|
-Supramolecule #1: C-reactive protein purified from human serum with calcium
| Supramolecule | Name: C-reactive protein purified from human serum with calcium type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 250 KDa |
-Macromolecule #1: C-reactive protein(1-205)
| Macromolecule | Name: C-reactive protein(1-205) / type: protein_or_peptide / ID: 1 / Number of copies: 10 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 25.061477 KDa |
| Sequence | String: MEKLLCFLVL TSLSHAFGQT DMSRKAFVFP KESDTSYVSL KAPLTKPLKA FTVCLHFYTE LSSTRGYSIF SYATKRQDNE ILIFWSKDI GYSFTVGGSE ILFEVPEVTV APVHICTSWE SASGIVEFWV DGKPRVRKSL KKGYTVGAEA SIILGQEQDS F GGNFEGSQ ...String: MEKLLCFLVL TSLSHAFGQT DMSRKAFVFP KESDTSYVSL KAPLTKPLKA FTVCLHFYTE LSSTRGYSIF SYATKRQDNE ILIFWSKDI GYSFTVGGSE ILFEVPEVTV APVHICTSWE SASGIVEFWV DGKPRVRKSL KKGYTVGAEA SIILGQEQDS F GGNFEGSQ SLVGDIGNVN MWDFVLSPDE INTIYLGGPF SPNVLNWRAL KYEVQGEVFT KPQLWP UniProtKB: C-reactive protein |
-Macromolecule #2: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 2 / Number of copies: 20 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.4 mg/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||
| Grid | Model: Quantifoil R0.6/1 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR | ||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 293 K / Instrument: FEI VITROBOT MARK IV / Details: Blot force, 0. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Average exposure time: 60.0 sec. / Average electron dose: 25.0 e/Å2 Details: Images were collected in movie mode @40 frames per second. Total of 25 frames were saved. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 130841 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.8 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)