[English] 日本語
 Yorodumi
Yorodumi- EMDB-35706: Cryo-EM structure of GIPR splice variant 1 (SV1) in complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of GIPR splice variant 1 (SV1) in complex with Gs protein | |||||||||||||||||||||
 Map data Map data | ||||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | Cryo-electron microscopy / G protein-coupled receptor / splice variant / receptor activation. / STRUCTURAL PROTEIN | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationgastric inhibitory peptide receptor activity / glucagon family peptide binding / gastric inhibitory peptide signaling pathway / endocrine pancreas development / desensitization of G protein-coupled receptor signaling pathway / sensory perception of chemical stimulus / response to fatty acid / mu-type opioid receptor binding / corticotropin-releasing hormone receptor 1 binding / G-protein activation ...gastric inhibitory peptide receptor activity / glucagon family peptide binding / gastric inhibitory peptide signaling pathway / endocrine pancreas development / desensitization of G protein-coupled receptor signaling pathway / sensory perception of chemical stimulus / response to fatty acid / mu-type opioid receptor binding / corticotropin-releasing hormone receptor 1 binding / G-protein activation / Activation of the phototransduction cascade / Glucagon-type ligand receptors / Thromboxane signalling through TP receptor / Sensory perception of sweet, bitter, and umami (glutamate) taste / G beta:gamma signalling through PI3Kgamma / G beta:gamma signalling through CDC42 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Ca2+ pathway / G alpha (z) signalling events / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / Adrenaline,noradrenaline inhibits insulin secretion / G protein-coupled peptide receptor activity / ADP signalling through P2Y purinoceptor 12 / G alpha (q) signalling events / beta-2 adrenergic receptor binding / G alpha (i) signalling events / Thrombin signalling through proteinase activated receptors (PARs) / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (12/13) signalling events / Glucagon-type ligand receptors / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Adrenaline,noradrenaline inhibits insulin secretion / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Thrombin signalling through proteinase activated receptors (PARs) / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / photoreceptor outer segment membrane / G alpha (q) signalling events / spectrin binding / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Vasopressin regulates renal water homeostasis via Aquaporins / alkylglycerophosphoethanolamine phosphodiesterase activity / peptide hormone binding / photoreceptor outer segment / D1 dopamine receptor binding / response to glucose / response to axon injury / activation of adenylate cyclase activity / adenylate cyclase-activating adrenergic receptor signaling pathway / insulin-like growth factor receptor binding / cardiac muscle cell apoptotic process / photoreceptor inner segment / response to nutrient / regulation of insulin secretion / ionotropic glutamate receptor binding / adenylate cyclase activator activity / generation of precursor metabolites and energy / response to calcium ion / positive regulation of insulin secretion / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein beta/gamma-subunit complex binding / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Glucagon-type ligand receptors / transmembrane signaling receptor activity / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / sensory perception of taste / signaling receptor complex adaptor activity / positive regulation of cytosolic calcium ion concentration / retina development in camera-type eye / cell body / GTPase binding / cellular response to hypoxia / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / cell surface receptor signaling pathway / cell population proliferation / G protein-coupled receptor signaling pathway Similarity search - Function | |||||||||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   | |||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.23 Å | |||||||||||||||||||||
 Authors Authors | Zhao FH / Hang KN / Zhou QT / Shao LJ / Li H / Li WZ / Lin S / Dai AT / Cai XQ / Liu YY ...Zhao FH / Hang KN / Zhou QT / Shao LJ / Li H / Li WZ / Lin S / Dai AT / Cai XQ / Liu YY / Xu YN / Feng WB / Yang DH / Wang MW | |||||||||||||||||||||
| Funding support |  China, 6 items China, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: Molecular basis of signal transduction mediated by the human GIPR splice variants. Authors: Fenghui Zhao / Kaini Hang / Qingtong Zhou / Lijun Shao / Hao Li / Wenzhuo Li / Shi Lin / Antao Dai / Xiaoqing Cai / Yanyun Liu / Yingna Xu / Wenbo Feng / Dehua Yang / Ming-Wei Wang /   Abstract: Glucose-dependent insulinotropic polypeptide receptor (GIPR) is a potential drug target for metabolic disorders. It works with glucagon-like peptide-1 receptor and glucagon receptor in humans to ...Glucose-dependent insulinotropic polypeptide receptor (GIPR) is a potential drug target for metabolic disorders. It works with glucagon-like peptide-1 receptor and glucagon receptor in humans to maintain glucose homeostasis. Unlike the other two receptors, GIPR has at least 13 reported splice variants (SVs), more than half of which have sequence variations at either C or N terminus. To explore their roles in endogenous peptide-mediated GIPR signaling, we determined the cryoelectron microscopy (cryo-EM) structures of the two N terminus-altered SVs (referred as GIPR-202 and GIPR-209 in the Ensembl database, SV1 and SV2 here, respectively) and investigated the outcome of coexpressing each of them in question with GIPR in HEK293T cells with respect to ligand binding, receptor expression, cAMP (adenosine 3,5-cyclic monophosphate) accumulation, β-arrestin recruitment, and cell surface localization. It was found that while both N terminus-altered SVs of GIPR neither bound to the hormone nor elicited signal transduction per se, they suppressed ligand binding and cAMP accumulation of GIPR. Meanwhile, SV1 reduced GIPR-mediated β-arrestin 2 responses. The cryo-EM structures of SV1 and SV2 showed that they reorganized the extracellular halves of transmembrane helices 1, 6, and 7 and extracellular loops 2 and 3 to adopt a ligand-binding pocket-occupied conformation, thereby losing binding ability to the peptide. The results suggest a form of signal bias that is constitutive and ligand-independent, thus expanding our knowledge of biased signaling beyond pharmacological manipulation (i.e., ligand specific) as well as constitutive and ligand-independent (e.g., SV1 of the growth hormone-releasing hormone receptor). | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35706.map.gz emd_35706.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35706-v30.xml emd-35706-v30.xml emd-35706.xml emd-35706.xml | 23 KB 23 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_35706.png emd_35706.png | 22.4 KB | ||
| Filedesc metadata |  emd-35706.cif.gz emd-35706.cif.gz | 7.1 KB | ||
| Others |  emd_35706_half_map_1.map.gz emd_35706_half_map_1.map.gz emd_35706_half_map_2.map.gz emd_35706_half_map_2.map.gz | 59.2 MB 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35706 http://ftp.pdbj.org/pub/emdb/structures/EMD-35706 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35706 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35706 | HTTPS FTP |
-Related structure data
| Related structure data |  8itlMC  8itmC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35706.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35706.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.071 Å | ||||||||||||||||||||||||||||||||||||
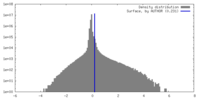
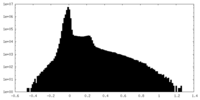
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_35706_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
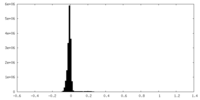
| Density Histograms |
-Half map: #1
| File | emd_35706_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
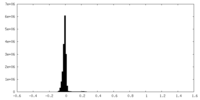
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of a splice variant of the GIPR(SV1) in complex...
| Entire | Name: Cryo-EM structure of a splice variant of the GIPR(SV1) in complex with Gs protein |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of a splice variant of the GIPR(SV1) in complex...
| Supramolecule | Name: Cryo-EM structure of a splice variant of the GIPR(SV1) in complex with Gs protein type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Gastric inhibitory polypeptide receptor
| Macromolecule | Name: Gastric inhibitory polypeptide receptor / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 42.252238 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: RAETGSKGQT AGELYQRWER YRRECQETLA AAEPPSVAAG FVLRQCGSDG QWGLWRDHTQ CENPEKNEAF LDQRLILERL QVMYTVGYS LSLATLLLAL LILSLFRRLH CTRNYIHINL FTSFMLRAAA ILSRDRLLPR PGPYLGDQAL ALWNQALAAC R TAQIVTQY ...String: RAETGSKGQT AGELYQRWER YRRECQETLA AAEPPSVAAG FVLRQCGSDG QWGLWRDHTQ CENPEKNEAF LDQRLILERL QVMYTVGYS LSLATLLLAL LILSLFRRLH CTRNYIHINL FTSFMLRAAA ILSRDRLLPR PGPYLGDQAL ALWNQALAAC R TAQIVTQY CVGANYTWLL VEGVYLHSLL VLVGGSEEGH FRYYLLLGWG APALFVIPWV IVRYLYENTQ CWERNEVKAI WW IIRTPIL MTILINFLIF IRILGILLSK LRTRQMRCRD YRLRLARSTL FLVPLLGVHE VVFAPVTEEQ ARGALRFAKL GFE IFLSSF QGFLVSVLYC FINKEVQSEI RRGWHHCRLR RSLGEEQR UniProtKB: Gastric inhibitory polypeptide receptor |
-Macromolecule #2: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
| Macromolecule | Name: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 45.727441 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKNTIVKQM RILHVNGFNG EGGEEDPQAA RSNSDGEKA TKVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV R ACYERSNE ...String: MGCLGNSKTE DQRNEEKAQR EANKKIEKQL QKDKQVYRAT HRLLLLGAGE SGKNTIVKQM RILHVNGFNG EGGEEDPQAA RSNSDGEKA TKVQDIKNNL KEAIETIVAA MSNLVPPVEL ANPENQFRVD YILSVMNVPD FDFPPEFYEH AKALWEDEGV R ACYERSNE YQLIDCAQYF LDKIDVIKQD DYVPSDQDLL RCRVLTSGIF ETKFQVDKVN FHMFDVGAQR DERRKWIQCF ND VTAIIFV VASSSYNMVI REDNQTNRLQ AALKLFDSIW NNKWLRDTSV ILFLNKQDLL AEKVLAGKSK IEDYFPEFAR YTT PEDATP EPGEDPRVTR AKYFIRDEFL RISTASGDGR HYCYPHFTCA VDTENIRRVF NDCRDIIQRM HLRQYELL UniProtKB: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 40.226992 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSLLQSELD QLRQEAEQLK NQIRDARKAC ADATLSQITN NIDPVGRIQM RTRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDS YTTNKVHAIP LRSSWVMTCA YAPSGNYVAC GGLDNICSIY NLKTREGNVR VSRELAGHTG YLSCCRFLDD N QIVTSSGD ...String: MGSLLQSELD QLRQEAEQLK NQIRDARKAC ADATLSQITN NIDPVGRIQM RTRRTLRGHL AKIYAMHWGT DSRLLVSASQ DGKLIIWDS YTTNKVHAIP LRSSWVMTCA YAPSGNYVAC GGLDNICSIY NLKTREGNVR VSRELAGHTG YLSCCRFLDD N QIVTSSGD TTCALWDIET GQQTTTFTGH TGDVMSLSLA PDTRLFVSGA CDASAKLWDV REGMCRQTFT GHESDINAIC FF PNGNAFA TGSDDATCRL FDLRADQELM TYSHDNIICG ITSVSFSKSG RLLLAGYDDF NCNVWDALKA DRAGVLAGHD NRV SCLGVT DDGMAVATGS WDSFLKIWNG SSGGGGSGGG GSSGVSGWRL FKKIS UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: Nanobody-35
| Macromolecule | Name: Nanobody-35 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 13.885439 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGGG LVQPGGSLRL SCAASGFTFS NYKMNWVRQA PGKGLEWVSD ISQSGASISY TGSVKGRFTI SRDNAKNTLY LQMNSLKPE DTAVYYCARC PAPFTRDCFD VTSTTYAYRG QGTQVTVSS |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 80.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source: OTHER |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)