+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of HCA3-Gi complex with acifran (local) | |||||||||
 Map data Map data | map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GPCR / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnicotinic acid receptor activity / Hydroxycarboxylic acid-binding receptors / electron transport chain / G protein-coupled receptor activity / cell junction / G alpha (i) signalling events / electron transfer activity / periplasmic space / iron ion binding / G protein-coupled receptor signaling pathway ...nicotinic acid receptor activity / Hydroxycarboxylic acid-binding receptors / electron transport chain / G protein-coupled receptor activity / cell junction / G alpha (i) signalling events / electron transfer activity / periplasmic space / iron ion binding / G protein-coupled receptor signaling pathway / heme binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.21 Å | |||||||||
 Authors Authors | Suzuki S / Nishikawa K / Suzuki H / Fujiyoshi Y | |||||||||
| Funding support |  Japan, 1 items Japan, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis of hydroxycarboxylic acid receptor signaling mechanisms through ligand binding. Authors: Shota Suzuki / Kotaro Tanaka / Kouki Nishikawa / Hiroshi Suzuki / Atsunori Oshima / Yoshinori Fujiyoshi /  Abstract: Hydroxycarboxylic acid receptors (HCA) are expressed in various tissues and immune cells. HCA2 and its agonist are thus important targets for treating inflammatory and metabolic disorders. Only ...Hydroxycarboxylic acid receptors (HCA) are expressed in various tissues and immune cells. HCA2 and its agonist are thus important targets for treating inflammatory and metabolic disorders. Only limited information is available, however, on the active-state binding of HCAs with agonists. Here, we present cryo-EM structures of human HCA2-Gi and HCA3-Gi signaling complexes binding with multiple compounds bound. Agonists were revealed to form a salt bridge with arginine, which is conserved in the HCA family, to activate these receptors. Extracellular regions of the receptors form a lid-like structure that covers the ligand-binding pocket. Although transmembrane (TM) 6 in HCAs undergoes dynamic conformational changes, ligands do not directly interact with amino acids in TM6, suggesting that indirect signaling induces a slight shift in TM6 to activate Gi proteins. Structural analyses of agonist-bound HCA2 and HCA3 together with mutagenesis and molecular dynamics simulation provide molecular insights into HCA ligand recognition and activation mechanisms. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_35447.map.gz emd_35447.map.gz | 34.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-35447-v30.xml emd-35447-v30.xml emd-35447.xml emd-35447.xml | 17.9 KB 17.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_35447_fsc.xml emd_35447_fsc.xml | 10.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_35447.png emd_35447.png | 25.1 KB | ||
| Masks |  emd_35447_msk_1.map emd_35447_msk_1.map | 37.4 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-35447.cif.gz emd-35447.cif.gz | 6.6 KB | ||
| Others |  emd_35447_half_map_1.map.gz emd_35447_half_map_1.map.gz emd_35447_half_map_2.map.gz emd_35447_half_map_2.map.gz | 34.7 MB 34.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-35447 http://ftp.pdbj.org/pub/emdb/structures/EMD-35447 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35447 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-35447 | HTTPS FTP |
-Related structure data
| Related structure data |  8ihkMC  8ihbC  8ihfC  8ihhC  8ihiC  8ihjC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_35447.map.gz / Format: CCP4 / Size: 37.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_35447.map.gz / Format: CCP4 / Size: 37.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.78 Å | ||||||||||||||||||||||||||||||||||||
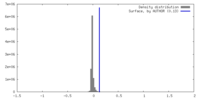
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_35447_msk_1.map emd_35447_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
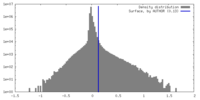
| Density Histograms |
-Half map: half map A
| File | emd_35447_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
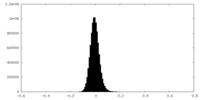
| Density Histograms |
-Half map: half map B
| File | emd_35447_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Multiprotein complex
| Entire | Name: Multiprotein complex |
|---|---|
| Components |
|
-Supramolecule #1: Multiprotein complex
| Supramolecule | Name: Multiprotein complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Soluble cytochrome b562,Hydroxycarboxylic acid receptor 3
| Macromolecule | Name: Soluble cytochrome b562,Hydroxycarboxylic acid receptor 3 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 60.749406 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDKADLEDN WETLNDNLKV IEKADNAAQV KDALTKMRAA ALDAQKATPP KLEDKSPDSP EMKDFRHGF DILVGQIDDA LKLANEGKVK EAQAAAEQLK TTRNAYIQKY LNRHHLQDHF LEIDKKNCCV FRDDFIAKVL P PVLGLEFI ...String: MKTIIALSYI FCLVFADYKD DDDKADLEDN WETLNDNLKV IEKADNAAQV KDALTKMRAA ALDAQKATPP KLEDKSPDSP EMKDFRHGF DILVGQIDDA LKLANEGKVK EAQAAAEQLK TTRNAYIQKY LNRHHLQDHF LEIDKKNCCV FRDDFIAKVL P PVLGLEFI FGLLGNGLAL WIFCFHLKSW KSSRIFLFNL AVADFLLIIC LPFVMDYYVR RSDWKFGDIP CRLVLFMFAM NR QGSIIFL TVVAVDRYFR VVHPHHALNK ISNWTAAIIS CLLWGITVGL TVHLLKKKLL IQNGTANVCI SFSICHTFRW HEA MFLLEF FLPLGIILFC SARIIWSLRQ RQMDRHAKIK RAITFIMVVA IVFVICFLPS VVVRIHIFWL LHTSGTQNCE VYRS VDLAF FITLSFTYMN SMLDPVVYYF SSPSFPNFFS TLINRCLQRK ITGEPDNNRS TSVELTGDPN KTRGAPEALI ANSGE PWSP SYLGPTSNNH SKKGHCHQEP ASLEKQLGCC IEENLYFQGS HHHHHH UniProtKB: Soluble cytochrome b562, Hydroxycarboxylic acid receptor 3 |
-Macromolecule #2: (5~{S})-5-methyl-4-oxidanylidene-5-phenyl-furan-2-carboxylic acid
| Macromolecule | Name: (5~{S})-5-methyl-4-oxidanylidene-5-phenyl-furan-2-carboxylic acid type: ligand / ID: 2 / Number of copies: 1 / Formula: P9X |
|---|---|
| Molecular weight | Theoretical: 218.205 Da |
| Chemical component information | 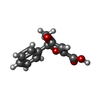 ChemComp-P9X: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 15 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K / Instrument: FEI VITROBOT MARK IV |
| Details | This sample was monodisperse |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 49.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)