+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Endothelin1-bound ETBR-Gq complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ET1 / ETAR / Gq / scFv16 / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationenteric smooth muscle cell differentiation / response to endothelin / : / negative regulation of neuron maturation / endothelin A receptor binding / chordate pharynx development / Oplophorus-luciferin 2-monooxygenase / Oplophorus-luciferin 2-monooxygenase activity / rhythmic excitation / neuroblast migration ...enteric smooth muscle cell differentiation / response to endothelin / : / negative regulation of neuron maturation / endothelin A receptor binding / chordate pharynx development / Oplophorus-luciferin 2-monooxygenase / Oplophorus-luciferin 2-monooxygenase activity / rhythmic excitation / neuroblast migration / negative regulation of phospholipase C/protein kinase C signal transduction / endothelin receptor activity / peptide hormone secretion / endothelin B receptor binding / aldosterone metabolic process / cellular response to human chorionic gonadotropin stimulus / meiotic cell cycle process involved in oocyte maturation / semaphorin-plexin signaling pathway involved in axon guidance / positive regulation of artery morphogenesis / histamine secretion / neural crest cell fate commitment / regulation of fever generation / vein smooth muscle contraction / glomerular endothelium development / response to prostaglandin F / sympathetic neuron axon guidance / positive regulation of penile erection / positive regulation of sarcomere organization / noradrenergic neuron differentiation / positive regulation of chemokine-mediated signaling pathway / leukocyte activation / phospholipase D-activating G protein-coupled receptor signaling pathway / maternal process involved in parturition / rough endoplasmic reticulum lumen / posterior midgut development / body fluid secretion / pharyngeal arch artery morphogenesis / regulation of D-glucose transmembrane transport / endothelin receptor signaling pathway involved in heart process / positive regulation of odontogenesis / epithelial fluid transport / cardiac neural crest cell migration involved in outflow tract morphogenesis / heparin proteoglycan metabolic process / negative regulation of hormone secretion / response to ozone / Weibel-Palade body / podocyte differentiation / endothelin receptor signaling pathway / positive regulation of cation channel activity / developmental pigmentation / positive regulation of cell growth involved in cardiac muscle cell development / renal sodium excretion / response to sodium phosphate / response to leptin / axonogenesis involved in innervation / glomerular filtration / enteric nervous system development / renin secretion into blood stream / positive regulation of smooth muscle contraction / renal sodium ion absorption / renal albumin absorption / cellular response to follicle-stimulating hormone stimulus / artery smooth muscle contraction / positive regulation of prostaglandin secretion / protein transmembrane transport / cellular response to luteinizing hormone stimulus / regulation of pH / cellular response to mineralocorticoid stimulus / respiratory gaseous exchange by respiratory system / melanocyte differentiation / vasoconstriction / positive regulation of renal sodium excretion / basal part of cell / peripheral nervous system development / type 1 angiotensin receptor binding / response to salt / negative regulation of adenylate cyclase activity / positive regulation of hormone secretion / regulation of systemic arterial blood pressure by endothelin / regulation of epithelial cell proliferation / dorsal/ventral pattern formation / cellular response to toxic substance / embryonic heart tube development / axon extension / cellular response to fatty acid / cartilage development / establishment of endothelial barrier / prostaglandin biosynthetic process / positive regulation of neutrophil chemotaxis / positive regulation of urine volume / signal transduction involved in regulation of gene expression / superoxide anion generation / negative regulation of protein metabolic process / neural crest cell migration / : / cellular response to glucocorticoid stimulus / nitric oxide transport / response to pain / middle ear morphogenesis / branching involved in blood vessel morphogenesis Similarity search - Function | |||||||||
| Biological species |  Homo (humans) / Homo (humans) /  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Yuan Q / Jiang Y / Xu HE / Ji Y / Duan J | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis of peptide recognition and activation of endothelin receptors. Authors: Yujie Ji / Jia Duan / Qingning Yuan / Xinheng He / Gong Yang / Shengnan Zhu / Kai Wu / Wen Hu / Tianyu Gao / Xi Cheng / Hualiang Jiang / H Eric Xu / Yi Jiang /  Abstract: Endothelin system comprises three endogenous 21-amino-acid peptide ligands endothelin-1, -2, and -3 (ET-1/2/3), and two G protein-coupled receptor (GPCR) subtypes-endothelin receptor A (ETR) and B ...Endothelin system comprises three endogenous 21-amino-acid peptide ligands endothelin-1, -2, and -3 (ET-1/2/3), and two G protein-coupled receptor (GPCR) subtypes-endothelin receptor A (ETR) and B (ETR). Since ET-1, the first endothelin, was identified in 1988 as one of the most potent endothelial cell-derived vasoconstrictor peptides with long-lasting actions, the endothelin system has attracted extensive attention due to its critical role in vasoregulation and close relevance in cardiovascular-related diseases. Here we present three cryo-electron microscopy structures of ETR and ETR bound to ET-1 and ETR bound to the selective peptide IRL1620. These structures reveal a highly conserved recognition mode of ET-1 and characterize the ligand selectivity by ETRs. They also present several conformation features of the active ETRs, thus revealing a specific activation mechanism. Together, these findings deepen our understanding of endothelin system regulation and offer an opportunity to design selective drugs targeting specific ETR subtypes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34667.map.gz emd_34667.map.gz | 57.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34667-v30.xml emd-34667-v30.xml emd-34667.xml emd-34667.xml | 23 KB 23 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_34667.png emd_34667.png | 42.3 KB | ||
| Filedesc metadata |  emd-34667.cif.gz emd-34667.cif.gz | 7.3 KB | ||
| Others |  emd_34667_half_map_1.map.gz emd_34667_half_map_1.map.gz emd_34667_half_map_2.map.gz emd_34667_half_map_2.map.gz | 49.4 MB 49.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34667 http://ftp.pdbj.org/pub/emdb/structures/EMD-34667 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34667 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34667 | HTTPS FTP |
-Related structure data
| Related structure data |  8hcxMC  8hbdC  8hcqC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34667.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34667.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34667_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
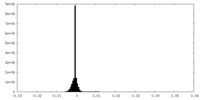
| Density Histograms |
-Half map: #1
| File | emd_34667_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
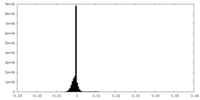
| Density Histograms |
- Sample components
Sample components
-Entire : ET1-ETAR-Gq-scFv16 complex
| Entire | Name: ET1-ETAR-Gq-scFv16 complex |
|---|---|
| Components |
|
-Supramolecule #1: ET1-ETAR-Gq-scFv16 complex
| Supramolecule | Name: ET1-ETAR-Gq-scFv16 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo (humans) Homo (humans) |
-Macromolecule #1: Guanine nucleotide-binding protein G(q) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(q) subunit alpha-1 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 28.084832 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSTVSAEDK AAAERSKMID KNLREDGEKA RRTLRLLLLG ADNSGKSTIV KQMRILHGGS GGSGGTSGIF ETKFQVDKVN FHMFDVGGQ RDERRKWIQC FNDVTAIIFV VDSSDYNRLQ EALNDFKSIW NNRWLRTISV ILFLNKQDLL AEKVLAGKSK I EDYFPEFA ...String: MGSTVSAEDK AAAERSKMID KNLREDGEKA RRTLRLLLLG ADNSGKSTIV KQMRILHGGS GGSGGTSGIF ETKFQVDKVN FHMFDVGGQ RDERRKWIQC FNDVTAIIFV VDSSDYNRLQ EALNDFKSIW NNRWLRTISV ILFLNKQDLL AEKVLAGKSK I EDYFPEFA RYTTPEDATP EPGEDPRVTR AKYFIRKEFV DISTASGDGR HICYPHFTCA VDTENARRIF NDCKDIILQM NL REYNLV |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.055867 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI ...String: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI VTSSGDTTCA LWDIETGQQT TTFTGHTGDV MSLSLAPDTR LFVSGACDAS AKLWDVREGM CRQTFTGHES DI NAICFFP NGNAFATGSD DATCRLFDLR ADQELMTYSH DNIICGITSV SFSKSGRLLL AGYDDFNCNV WDALKADRAG VLA GHDNRV SCLGVTDDGM AVATGSWDSF LKIWNGSSGG GGSGGGGSSG VSGWRLFKKI S UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Endothelin receptor type B,Oplophorus-luciferin 2-monooxygenase c...
| Macromolecule | Name: Endothelin receptor type B,Oplophorus-luciferin 2-monooxygenase catalytic subunit chimera type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: Oplophorus-luciferin 2-monooxygenase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 67.88232 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDSKGSSQKG SRLLLLLVVS NLLLCQGVVS DYKDDDDVDH HHHHHHHEER GFPPDRATPL LQTAEIMTPP TKTLWPKGSN ASLARSLAP AEVPKGDRTA GSPPRTISPP PCQGPIEIKE TFKYINTVVS CLVFVLGIIG NSTLLRIIYK NKCMRNGPNI L IASLALGD ...String: MDSKGSSQKG SRLLLLLVVS NLLLCQGVVS DYKDDDDVDH HHHHHHHEER GFPPDRATPL LQTAEIMTPP TKTLWPKGSN ASLARSLAP AEVPKGDRTA GSPPRTISPP PCQGPIEIKE TFKYINTVVS CLVFVLGIIG NSTLLRIIYK NKCMRNGPNI L IASLALGD LLHIVIDIPI NVYKLLAEDW PFGAEMCKLV PFIQKASVGI TVLSLCALSI DRYRAVASWS RIKGIGVPKW TA VEIVLIW VVSVVLAVPE AIGFDIITMD YKGSYLRICL LHPVQKTAFM QFYKTAKDWW LFSFYFCLPL AITAFFYTLM TCE MLRKKS GMQIALNDHL KQRREVAKTV FCLVLVFALC WLPLHLSRIL KLTLYNQNDP NRCELLSFLL VLDYIGINMA SLNS CINPI ALYLVSKRFK NCFKSCLCCW CQSFEEKQSL EEKQSCLKFK VFTLEDFVGD WEQTAAYNLD QVLEQGGVSS LLQNL AVSV TPIQRIVRSG ENALKIDIHV IIPYEGLSAD QMAQIEEVFK VVYPVDDHHF KVILPYGTLV IDGVTPNMLN YFGRPY EGI AVFDGKKITV TGTLWNGNKI IDERLITPDG SMLFRVTINS UniProtKB: Endothelin receptor type B, Oplophorus-luciferin 2-monooxygenase catalytic subunit |
-Macromolecule #4: Endothelin-1
| Macromolecule | Name: Endothelin-1 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 2.497951 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: CSCSSLMDKE CVYFCHLDII W UniProtKB: Endothelin-1 |
-Macromolecule #5: scFv16
| Macromolecule | Name: scFv16 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 30.363043 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFAVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSAG G GGSGGGGS ...String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFAVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSAG G GGSGGGGS GGGGSADIVM TQATSSVPVT PGESVSISCR SSKSLLHSNG NTYLYWFLQR PGQSPQLLIY RMSNLASGVP DR FSGSGSG TAFTLTISRL EAEDVGVYYC MQHLEYPLTF GAGTKLEL |
-Macromolecule #6: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)