+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of somatostatin receptor 2 bound with octreotide. | |||||||||
 Map data Map data | Cryo-EM density map of octreotide bound SSTR2-DNGi complex. | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationsomatostatin receptor activity / Olfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Activation of the phototransduction cascade / neuropeptide binding / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor ...somatostatin receptor activity / Olfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Activation of the phototransduction cascade / neuropeptide binding / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (12/13) signalling events / Glucagon-type ligand receptors / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Adrenaline,noradrenaline inhibits insulin secretion / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / cellular response to glucocorticoid stimulus / Thrombin signalling through proteinase activated receptors (PARs) / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / G alpha (q) signalling events / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Vasopressin regulates renal water homeostasis via Aquaporins / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / neuropeptide signaling pathway / adenylate cyclase inhibitor activity / positive regulation of protein localization to cell cortex / T cell migration / Adenylate cyclase inhibitory pathway / D2 dopamine receptor binding / response to prostaglandin E / adenylate cyclase regulator activity / G protein-coupled serotonin receptor binding / adenylate cyclase-inhibiting serotonin receptor signaling pathway / cellular response to forskolin / Peptide ligand-binding receptors / regulation of mitotic spindle organization / PDZ domain binding / Regulation of insulin secretion / cellular response to estradiol stimulus / positive regulation of cholesterol biosynthetic process / negative regulation of insulin secretion / G protein-coupled receptor binding / response to peptide hormone / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / centriolar satellite / G-protein beta/gamma-subunit complex binding / ADP signalling through P2Y purinoceptor 12 / photoreceptor disc membrane / Adrenaline,noradrenaline inhibits insulin secretion / GDP binding / G alpha (z) signalling events / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / adenylate cyclase-activating dopamine receptor signaling pathway / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / sensory perception of taste / signaling receptor complex adaptor activity / G protein activity / retina development in camera-type eye / GTPase binding / midbody / cell cortex / G alpha (i) signalling events / G alpha (s) signalling events / phospholipase C-activating G protein-coupled receptor signaling pathway / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / Extra-nuclear estrogen signaling / cell population proliferation / neuron projection / ciliary basal body / G protein-coupled receptor signaling pathway / negative regulation of cell population proliferation / lysosomal membrane / cell division / GTPase activity / synapse / centrosome / GTP binding / protein-containing complex binding / nucleolus / magnesium ion binding / Golgi apparatus / extracellular exosome / nucleoplasm / membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) / Homo sapiens (human) / synthetic construct (others) /   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.97 Å | |||||||||
 Authors Authors | Bo Q / Yang F / Li YG / Meng XY / Zhang HH / Zhou YX / Ling SL / Sun DM / Lv P / Liu L ...Bo Q / Yang F / Li YG / Meng XY / Zhang HH / Zhou YX / Ling SL / Sun DM / Lv P / Liu L / Shi P / Tian CL | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Discov / Year: 2022 Journal: Cell Discov / Year: 2022Title: Structural insights into the activation of somatostatin receptor 2 by cyclic SST analogues. Authors: Qing Bo / Fan Yang / Yingge Li / Xianyu Meng / Huanhuan Zhang / Yingxin Zhou / Shenglong Ling / Demeng Sun / Pei Lv / Lei Liu / Pan Shi / Changlin Tian /  Abstract: The endogenous cyclic tetradecapeptide SST14 was reported to stimulate all five somatostatin receptors (SSTR1-5) for hormone release, neurotransmission, cell growth arrest and cancer suppression. Two ...The endogenous cyclic tetradecapeptide SST14 was reported to stimulate all five somatostatin receptors (SSTR1-5) for hormone release, neurotransmission, cell growth arrest and cancer suppression. Two SST14-derived short cyclic SST analogues (lanreotide or octreotide) with improved stability and longer lifetime were developed as drugs to preferentially activate SSTR2 and treat acromegalia and neuroendocrine tumors. Here, cryo-EM structures of the human SSTR2-Gi complex bound with SST14, octreotide or lanreotide were determined at resolutions of 2.85 Å, 2.97 Å, and 2.87 Å, respectively. Structural and functional analysis revealed that interactions between β-turn residues in SST analogues and transmembrane SSTR2 residues in the ligand-binding pocket are crucial for receptor binding and functional stimulation of the two SST14-derived cyclic octapeptides. Additionally, Q102, N276, and F294 could be responsible for the selectivity of lanreotide or octreotide for SSTR2 over SSTR1 or SSTR4. These results provide valuable insights into further rational development of SST analogue drugs targeting SSTR2. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33099.map.gz emd_33099.map.gz | 28.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33099-v30.xml emd-33099-v30.xml emd-33099.xml emd-33099.xml | 20.2 KB 20.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_33099.png emd_33099.png | 87.3 KB | ||
| Others |  emd_33099_half_map_1.map.gz emd_33099_half_map_1.map.gz emd_33099_half_map_2.map.gz emd_33099_half_map_2.map.gz | 28.3 MB 28.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33099 http://ftp.pdbj.org/pub/emdb/structures/EMD-33099 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33099 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33099 | HTTPS FTP |
-Related structure data
| Related structure data |  7xauMC  7xatC  7xavC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33099.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33099.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM density map of octreotide bound SSTR2-DNGi complex. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
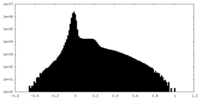
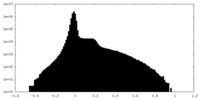
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Cryo-EM density half map B of octreotide bound SSTR2-DNGi complex.
| File | emd_33099_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM density half map B of octreotide bound SSTR2-DNGi complex. | ||||||||||||
| Projections & Slices |
| ||||||||||||
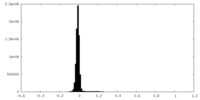
| Density Histograms |
-Half map: Cryo-EM density half map A of octreotide bound SSTR2-DNGi complex.
| File | emd_33099_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM density half map A of octreotide bound SSTR2-DNGi complex. | ||||||||||||
| Projections & Slices |
| ||||||||||||
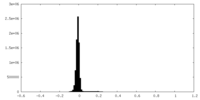
| Density Histograms |
- Sample components
Sample components
-Entire : The SST anologue octreotide bound somatostatin receptor 2 and Gi ...
| Entire | Name: The SST anologue octreotide bound somatostatin receptor 2 and Gi heterotrimer complex |
|---|---|
| Components |
|
-Supramolecule #1: The SST anologue octreotide bound somatostatin receptor 2 and Gi ...
| Supramolecule | Name: The SST anologue octreotide bound somatostatin receptor 2 and Gi heterotrimer complex type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Somatostatin receptor type 2,LargeBit
| Macromolecule | Name: Somatostatin receptor type 2,LargeBit / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 63.274395 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDKGSGSHH HHHHHHHHLE VLFQGPMDMA DEPLNGSHTW LSIPFDLNGS VVSTNTSNQT EPYYDLTSN AVLTFIYFVV CIIGLCGNTL VIYVILRYAK MKTITNIYIL NLAIADELFM LGLPFLAMQV ALVHWPFGKA I CRVVMTVD ...String: MKTIIALSYI FCLVFADYKD DDDKGSGSHH HHHHHHHHLE VLFQGPMDMA DEPLNGSHTW LSIPFDLNGS VVSTNTSNQT EPYYDLTSN AVLTFIYFVV CIIGLCGNTL VIYVILRYAK MKTITNIYIL NLAIADELFM LGLPFLAMQV ALVHWPFGKA I CRVVMTVD GINQFTSIFC LTVMSIDRYL AVVHPIKSAK WRRPRTAKMI TMAVWGVSLL VILPIMIYAG LRSNQWGRSS CT INWPGES GAWYTGFIIY TFILGFLVPL TIICLCYLFI IIKVKSSGIR VGSSKRKKSE KKVTRMVSIV VAVFIFCWLP FYI FNVSSV SMAISPTPAL KGMFDFVVVL TYANSCANPI LYAFLSDNFK KSFQNVLCLV KVSGTDDGER SDSKQDKSRL NETT ETQRT VFTLEDFVGD WEQTAAYNLD QVLEQGGVSS LLQNLAVSVT PIQRIVRSGE NALKIDIHVI IPYEGLSADQ MAQIE EVFK VVYPVDDHHF KVILPYGTLV IDGVTPNMLN YFGRPYEGIA VFDGKKITVT GTLWNGNKII DERLITPDGS MLFRVT INS |
-Macromolecule #2: Guanine nucleotide-binding protein G(i) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.472082 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKNTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMID RNLREDGEKA AREVKLLLLG AGESGKNTIV KQMKIIHEAG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTTGIVETH FTFKDLHFKM FDVGAQRSER KKWIHCFEGV TAIIFCVALS DYDLVLAEDE EM NRMHESM KLFDSICNNK WFTDTSIILF LNKKDLFEEK IKKSPLTICY PEYAGSNTYE EAAAYIQCQF EDLNKRKDTK EIY THFTCS TDTKNVQFVF DAVTDVIIKN NLKDCGLF |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 38.975578 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI ...String: MHHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT REGNVRVSRE LAGHTGYLSC C RFLDDNQI VTSSGDTTCA LWDIETGQQT TTFTGHTGDV MSLSLAPDTR LFVSGACDAS AKLWDVREGM CRQTFTGHES DI NAICFFP NGNAFATGSD DATCRLFDLR ADQELMTYSH DNIICGITSV SFSKSGRLLL AGYDDFNCNV WDALKADRAG VLA GHDNRV SCLGVTDDGM AVATGSWDSF LKIWNGSS |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L |
-Macromolecule #5: ScFv16
| Macromolecule | Name: ScFv16 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 32.768484 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFDVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSSG G GGSGGGGS ...String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFDVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSSG G GGSGGGGS GGGGSSDIVM TQATSSVPVT PGESVSISCR SSKSLLHSNG NTYLYWFLQR PGQSPQLLIY RMSNLASGVP DR FSGSGSG TAFTLTISRL EAEDVGVYYC MQHLEYPLTF GAGTKLELVD ENLYFQGASH HHHHHHH |
-Macromolecule #6: Octreotide
| Macromolecule | Name: Octreotide / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 1.022263 KDa |
| Sequence | String: (DPN)CF(DTR)KTC(THO) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 55.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: DIFFRACTION / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER / Details: Alphafold |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.97 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 666575 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)