[English] 日本語
 Yorodumi
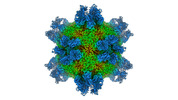
Yorodumi- EMDB-32998: Cryo-EM structure of Coxsackievirus B1 mature virion in complex w... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Coxsackievirus B1 mature virion in complex with nAb 8A10 (classified from CVB1 mature virion in complex with 8A10 and 2E6) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell / symbiont-mediated suppression of host gene expression ...symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell / symbiont-mediated suppression of host gene expression / nucleoside-triphosphate phosphatase / channel activity / viral capsid / monoatomic ion transmembrane transport / host cell cytoplasm / DNA replication / RNA helicase activity / induction by virus of host autophagy / symbiont entry into host cell / RNA-directed RNA polymerase / viral RNA genome replication / cysteine-type endopeptidase activity / RNA-dependent RNA polymerase activity / virus-mediated perturbation of host defense response / DNA-templated transcription / host cell nucleus / virion attachment to host cell / structural molecule activity / ATP hydrolysis activity / proteolysis / RNA binding / ATP binding / metal ion binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Coxsackievirus B1 / Coxsackievirus B1 /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.02 Å | |||||||||
 Authors Authors | Zheng Q / Zhu R / Sun H / Cheng T / Li S / Xia N | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Cell Host Microbe / Year: 2022 Journal: Cell Host Microbe / Year: 2022Title: Structural basis for the synergistic neutralization of coxsackievirus B1 by a triple-antibody cocktail. Authors: Qingbing Zheng / Rui Zhu / Zhichao Yin / Longfa Xu / Hui Sun / Hai Yu / Yuanyuan Wu / Yichao Jiang / Qiongzi Huang / Yang Huang / Dongqing Zhang / Liqin Liu / Hongwei Yang / Maozhou He / ...Authors: Qingbing Zheng / Rui Zhu / Zhichao Yin / Longfa Xu / Hui Sun / Hai Yu / Yuanyuan Wu / Yichao Jiang / Qiongzi Huang / Yang Huang / Dongqing Zhang / Liqin Liu / Hongwei Yang / Maozhou He / Zhenhong Zhou / Yanan Jiang / Zhenqin Chen / Huan Zhao / Yuqiong Que / Zhibo Kong / Lizhi Zhou / Tingting Li / Jun Zhang / Wenxin Luo / Ying Gu / Tong Cheng / Shaowei Li / Ningshao Xia /  Abstract: Coxsackievirus B1 (CVB1) is an emerging pathogen associated with severe neonatal diseases including aseptic meningitis, myocarditis, and pancreatitis and also with the development of type 1 diabetes. ...Coxsackievirus B1 (CVB1) is an emerging pathogen associated with severe neonatal diseases including aseptic meningitis, myocarditis, and pancreatitis and also with the development of type 1 diabetes. We characterize the binding and therapeutic efficacies of three CVB1-specific neutralizing antibodies (nAbs) identified for their ability to inhibit host receptor engagement. High-resolution cryo-EM structures showed that these antibodies recognize different epitopes but with an overlapping region in the capsid VP2 protein and specifically the highly variable EF loop. Moreover, they perturb capsid-receptor interactions by binding various viral particle forms. Antibody combinations achieve synergetic neutralization via a stepwise capsid transition and virion disruption, indicating dynamic changes in the virion in response to multiple nAbs targeting the receptor-binding site. Furthermore, this three-antibody cocktail protects against lethal challenge in neonatal mice and limits pancreatitis and viral replication in a non-obese diabetic mouse model. These results illustrate the utility of nAbs for rational design of therapeutics against picornaviruses such as CVB. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32998.map.gz emd_32998.map.gz | 630.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32998-v30.xml emd-32998-v30.xml emd-32998.xml emd-32998.xml | 15.3 KB 15.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32998.png emd_32998.png | 73.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32998 http://ftp.pdbj.org/pub/emdb/structures/EMD-32998 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32998 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32998 | HTTPS FTP |
-Validation report
| Summary document |  emd_32998_validation.pdf.gz emd_32998_validation.pdf.gz | 529.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32998_full_validation.pdf.gz emd_32998_full_validation.pdf.gz | 529.5 KB | Display | |
| Data in XML |  emd_32998_validation.xml.gz emd_32998_validation.xml.gz | 8.4 KB | Display | |
| Data in CIF |  emd_32998_validation.cif.gz emd_32998_validation.cif.gz | 9.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32998 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32998 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32998 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32998 | HTTPS FTP |
-Related structure data
| Related structure data |  7x40MC  7x2gC  7x2iC  7x2oC  7x2tC  7x2wC  7x35C  7x37C  7x38C  7x3cC  7x3dC  7x3eC  7x3fC  7x3yC  7x42C  7x46C  7x47C  7x49C  7x4kC  7x4mC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32998.map.gz / Format: CCP4 / Size: 669.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32998.map.gz / Format: CCP4 / Size: 669.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.12 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Cryo-EM structure of Coxsackievirus B1 mature virion in complex w...
| Entire | Name: Cryo-EM structure of Coxsackievirus B1 mature virion in complex with nAb 8A10 (classified from CVB1 mature virion in complex with 8A10 and 2E6) |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of Coxsackievirus B1 mature virion in complex w...
| Supramolecule | Name: Cryo-EM structure of Coxsackievirus B1 mature virion in complex with nAb 8A10 (classified from CVB1 mature virion in complex with 8A10 and 2E6) type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: #1-#6 |
|---|---|
| Source (natural) | Organism:  Coxsackievirus B1 Coxsackievirus B1 |
-Macromolecule #1: 8A10 light chain
| Macromolecule | Name: 8A10 light chain / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 11.945163 KDa |
| Sequence | String: DIQMTQTKSS LSASLGDRVT ISCRASQDIS NYLNWYQQKP DGSVKLLIYY TSTLHSGVPS RFSGSGSGTD YSLTINSLEQ EDIATYFCQ QGNTFPFTFG GGTKLEIRR |
-Macromolecule #2: 8A10 heavy chain
| Macromolecule | Name: 8A10 heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 13.323863 KDa |
| Sequence | String: QVQLQQSAAE LARPGASVKM SCKASGYTFT TYTMHWVKQR PGQGLEWIGY INPSSRYTEY NQKFKDKTTL TADKSSSTAY MQLSSLTFE DSAVYYCARR SEADRFVYWG QGTLVTVSA |
-Macromolecule #3: Virion protein 1
| Macromolecule | Name: Virion protein 1 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Coxsackievirus B1 Coxsackievirus B1 |
| Molecular weight | Theoretical: 31.207117 KDa |
| Sequence | String: GPVEESVDRA VARVADTISS RPTNSESIPA LTAAETGHTS QVVPSDTMQT RHVKNYHSRS ESSIENFLCR SACVYYATYT NNSKKGFAE WVINTRQVAQ LRRKLELFTY LRFDLELTFV ITSAQQPSTA SSVDAPVQTH QIMYVPPGGP VPTKVKDYAW Q TSTNPSVF ...String: GPVEESVDRA VARVADTISS RPTNSESIPA LTAAETGHTS QVVPSDTMQT RHVKNYHSRS ESSIENFLCR SACVYYATYT NNSKKGFAE WVINTRQVAQ LRRKLELFTY LRFDLELTFV ITSAQQPSTA SSVDAPVQTH QIMYVPPGGP VPTKVKDYAW Q TSTNPSVF WTEGNAPPRM SIPFISIGNA YSCFYDGWTQ FSRNGVYGIN TLNNMGTLYM RHVNEAGQGP IKSTVRIYFK PK HVKAWVP RPPRLCQYEK QKNVNFSPIG VTTSRTDIIT T |
-Macromolecule #4: VP2
| Macromolecule | Name: VP2 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Coxsackievirus B1 Coxsackievirus B1 |
| Molecular weight | Theoretical: 29.122744 KDa |
| Sequence | String: SPSAEECGYS DRVRSITLGN STITTQECAN VVVGYGVWPE YLKDNEATAE DQPTQPDVAT CRFYTLESVQ WMKNSAGWWW KLPDALSQM GLFGQNMQYH YLGRTGYTIH VQCNASKFHQ GCLLVVCVPE AEMGCSNLNN TPEFSELSGG DSARMFTDTQ V GESNAKKV ...String: SPSAEECGYS DRVRSITLGN STITTQECAN VVVGYGVWPE YLKDNEATAE DQPTQPDVAT CRFYTLESVQ WMKNSAGWWW KLPDALSQM GLFGQNMQYH YLGRTGYTIH VQCNASKFHQ GCLLVVCVPE AEMGCSNLNN TPEFSELSGG DSARMFTDTQ V GESNAKKV QTAVWNAGMG VGVGNLTIFP HQWINLRTNN SATLVMPYIN SVPMDNMFRH NNLTLMIIPF VPLNYSEGSS PY VPITVTI APMCAEYNGL RLASNQ |
-Macromolecule #5: VP3
| Macromolecule | Name: VP3 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO / EC number: picornain 2A |
|---|---|
| Source (natural) | Organism:  Coxsackievirus B1 Coxsackievirus B1 |
| Molecular weight | Theoretical: 26.328764 KDa |
| Sequence | String: GLPVMTTPGS TQFLTSDDFQ SPSAMPQFDV TPEMQIPGRV NNLMEIAEVD SVVPVNNTED NVSSLKAYQI PVQSNSDNGK QVFGFPLQP GANNVLNRTL LGEILNYYTH WSGSIKLTFM FCGSAMATGK FLLAYSPPGA GVPKNRKDAM LGTHVIWDVG L QSSCVLCV ...String: GLPVMTTPGS TQFLTSDDFQ SPSAMPQFDV TPEMQIPGRV NNLMEIAEVD SVVPVNNTED NVSSLKAYQI PVQSNSDNGK QVFGFPLQP GANNVLNRTL LGEILNYYTH WSGSIKLTFM FCGSAMATGK FLLAYSPPGA GVPKNRKDAM LGTHVIWDVG L QSSCVLCV PWISQTHYRY VVEDEYTAAG YVTCWYQTNI VVPADVQSSC DILCFVSACN DFSVRMLKDT PFIRQDTFYQ |
-Macromolecule #6: Capsid protein VP4
| Macromolecule | Name: Capsid protein VP4 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Coxsackievirus B1 Coxsackievirus B1 |
| Molecular weight | Theoretical: 7.484246 KDa |
| Sequence | String: MGAQVSTQKT GAHETGLNAS GNSVIHYTNI NYYKDAASNS ANRQDFTQDP GKFTEPVKDI MVKTMPALN |
-Macromolecule #7: PALMITIC ACID
| Macromolecule | Name: PALMITIC ACID / type: ligand / ID: 7 / Number of copies: 1 / Formula: PLM |
|---|---|
| Molecular weight | Theoretical: 256.424 Da |
| Chemical component information |  ChemComp-PLM: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F30 |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.3000000000000003 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.02 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 156492 |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















