[English] 日本語
 Yorodumi
Yorodumi- EMDB-3222: Structure of a Chaperone-Usher pilus reveals the molecular basis ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3222 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of a Chaperone-Usher pilus reveals the molecular basis of rod uncoilin | |||||||||
 Map data Map data | Reconstruction of P pilus | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | helical polymer / strand donation | |||||||||
| Function / homology | Fimbrial-type adhesion domain / Fimbrial protein / : / Fimbrial-type adhesion domain superfamily / cell adhesion involved in single-species biofilm formation / Adhesion domain superfamily / pilus / extracellular region / Pap fimbrial major pilin protein Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Hospenthal MK / Redzej A / Dodson K / Ukleja M / Frenz B / Hultgren SJ / DiMaio F / Egelman EH / Waksman G | |||||||||
 Citation Citation |  Journal: Cell / Year: 2016 Journal: Cell / Year: 2016Title: Structure of a Chaperone-Usher Pilus Reveals the Molecular Basis of Rod Uncoiling. Authors: Manuela K Hospenthal / Adam Redzej / Karen Dodson / Marta Ukleja / Brandon Frenz / Catarina Rodrigues / Scott J Hultgren / Frank DiMaio / Edward H Egelman / Gabriel Waksman /   Abstract: Types 1 and P pili are prototypical bacterial cell-surface appendages playing essential roles in mediating adhesion of bacteria to the urinary tract. These pili, assembled by the chaperone-usher ...Types 1 and P pili are prototypical bacterial cell-surface appendages playing essential roles in mediating adhesion of bacteria to the urinary tract. These pili, assembled by the chaperone-usher pathway, are polymers of pilus subunits assembling into two parts: a thin, short tip fibrillum at the top, mounted on a long pilus rod. The rod adopts a helical quaternary structure and is thought to play essential roles: its formation may drive pilus extrusion by preventing backsliding of the nascent growing pilus within the secretion pore; the rod also has striking spring-like properties, being able to uncoil and recoil depending on the intensity of shear forces generated by urine flow. Here, we present an atomic model of the P pilus generated from a 3.8 Å resolution cryo-electron microscopy reconstruction. This structure provides the molecular basis for the rod's remarkable mechanical properties and illuminates its role in pilus secretion. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3222.map.gz emd_3222.map.gz | 3.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3222-v30.xml emd-3222-v30.xml emd-3222.xml emd-3222.xml | 8.5 KB 8.5 KB | Display Display |  EMDB header EMDB header |
| Images |  EMD-3222_EMDB.jpg EMD-3222_EMDB.jpg | 113.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3222 http://ftp.pdbj.org/pub/emdb/structures/EMD-3222 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3222 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3222 | HTTPS FTP |
-Validation report
| Summary document |  emd_3222_validation.pdf.gz emd_3222_validation.pdf.gz | 310.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_3222_full_validation.pdf.gz emd_3222_full_validation.pdf.gz | 309.2 KB | Display | |
| Data in XML |  emd_3222_validation.xml.gz emd_3222_validation.xml.gz | 5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3222 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3222 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3222 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3222 | HTTPS FTP |
-Related structure data
| Related structure data |  5fluMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_3222.map.gz / Format: CCP4 / Size: 3.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3222.map.gz / Format: CCP4 / Size: 3.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of P pilus | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
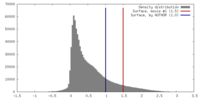
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : P pilus
| Entire | Name: P pilus |
|---|---|
| Components |
|
-Supramolecule #1000: P pilus
| Supramolecule | Name: P pilus / type: sample / ID: 1000 / Number unique components: 1 |
|---|
-Macromolecule #1: PapA
| Macromolecule | Name: PapA / type: protein_or_peptide / ID: 1 / Oligomeric state: helical polymer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
|---|
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Date | Jul 8, 2015 |
| Image recording | Category: CCD / Film or detector model: FEI FALCON II (4k x 4k) / Number real images: 90 / Average electron dose: 17 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | IHRSR |
|---|---|
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 7.7 Å Applied symmetry - Helical parameters - Δ&Phi: 109.8 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 3.8 Å / Resolution method: OTHER / Software - Name: Spider |
| CTF correction | Details: Each image |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)