+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30398 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of S.typhimurium flagellar LP ring | |||||||||
 Map data Map data | CryoEM structure of S.typhimurium flagellar LP ring | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationbacterial-type flagellum basal body, distal rod, L ring / bacterial-type flagellum basal body, distal rod, P ring / cytoskeletal motor activity / bacterial-type flagellum-dependent cell motility / cell outer membrane / outer membrane-bounded periplasmic space / structural molecule activity Similarity search - Function | |||||||||
| Biological species |  Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) / Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) /  Salmonella typhimurium (bacteria) Salmonella typhimurium (bacteria) | |||||||||
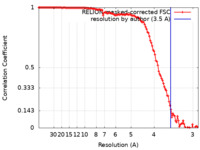
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Yamaguchi T / Makino F / Miyata T / Minamino T / Kato T / Namba K | |||||||||
| Funding support |  Japan, 2 items Japan, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Structure of the molecular bushing of the bacterial flagellar motor. Authors: Tomoko Yamaguchi / Fumiaki Makino / Tomoko Miyata / Tohru Minamino / Takayuki Kato / Keiichi Namba /  Abstract: The basal body of the bacterial flagellum is a rotary motor that consists of several rings (C, MS and LP) and a rod. The LP ring acts as a bushing supporting the distal rod for its rapid and stable ...The basal body of the bacterial flagellum is a rotary motor that consists of several rings (C, MS and LP) and a rod. The LP ring acts as a bushing supporting the distal rod for its rapid and stable rotation without much friction. Here, we use electron cryomicroscopy to describe the LP ring structure around the rod, at 3.5 Å resolution, from Salmonella Typhimurium. The structure shows 26-fold rotational symmetry and intricate intersubunit interactions of each subunit with up to six partners, which explains the structural stability. The inner surface is charged both positively and negatively. Positive charges on the P ring (the part of the LP ring that is embedded within the peptidoglycan layer) presumably play important roles in its initial assembly around the rod with a negatively charged surface. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30398.map.gz emd_30398.map.gz | 7.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30398-v30.xml emd-30398-v30.xml emd-30398.xml emd-30398.xml | 16.7 KB 16.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_30398_fsc.xml emd_30398_fsc.xml | 12 KB | Display |  FSC data file FSC data file |
| Images |  emd_30398.png emd_30398.png | 187.6 KB | ||
| Masks |  emd_30398_msk_1.map emd_30398_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30398 http://ftp.pdbj.org/pub/emdb/structures/EMD-30398 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30398 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30398 | HTTPS FTP |
-Validation report
| Summary document |  emd_30398_validation.pdf.gz emd_30398_validation.pdf.gz | 412.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_30398_full_validation.pdf.gz emd_30398_full_validation.pdf.gz | 412.5 KB | Display | |
| Data in XML |  emd_30398_validation.xml.gz emd_30398_validation.xml.gz | 12.7 KB | Display | |
| Data in CIF |  emd_30398_validation.cif.gz emd_30398_validation.cif.gz | 17.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30398 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30398 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30398 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30398 | HTTPS FTP |
-Related structure data
| Related structure data |  7clrMC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30398.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30398.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM structure of S.typhimurium flagellar LP ring | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.45 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_30398_msk_1.map emd_30398_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : LP ring
| Entire | Name: LP ring |
|---|---|
| Components |
|
-Supramolecule #1: LP ring
| Supramolecule | Name: LP ring / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Bushing of flagellar motor |
|---|---|
| Source (natural) | Organism:  Salmonella enterica subsp. enterica serovar Typhimurium (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium (bacteria)Strain: HK1002 |
-Macromolecule #1: Flagellar P-ring protein
| Macromolecule | Name: Flagellar P-ring protein / type: protein_or_peptide / ID: 1 / Number of copies: 26 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Salmonella typhimurium (bacteria) Salmonella typhimurium (bacteria) |
| Molecular weight | Theoretical: 38.194176 KDa |
| Sequence | String: MFKALAGIVL ALVATLAHAE RIRDLTSVQG VRENSLIGYG LVVGLDGTGD QTTQTPFTTQ TLNNMLSQLG ITVPTGTNMQ LKNVAAVMV TASYPPFARQ GQTIDVVVSS MGNAKSLRGG TLLMTPLKGV DSQVYALAQG NILVGGAGAS AGGSSVQVNQ L NGGRITNG ...String: MFKALAGIVL ALVATLAHAE RIRDLTSVQG VRENSLIGYG LVVGLDGTGD QTTQTPFTTQ TLNNMLSQLG ITVPTGTNMQ LKNVAAVMV TASYPPFARQ GQTIDVVVSS MGNAKSLRGG TLLMTPLKGV DSQVYALAQG NILVGGAGAS AGGSSVQVNQ L NGGRITNG AIIERELPTQ FGAGNTINLQ LNDEDFTMAQ QITDAINRAR GYGSATALDA RTVQVRVPSG NSSQVRFLAD IQ NMEVNVT PQDAKVVINS RTGSVVMNRE VTLDSCAVAQ GNLSVTVNRQ LNVNQPNTPF GGGQTVVTPQ TQIDLRQSGG SLQ SVRSSA NLNSVVRALN ALGATPMDLM SILQSMQSAG CLRAKLEII |
-Macromolecule #2: Flagellar L-ring protein
| Macromolecule | Name: Flagellar L-ring protein / type: protein_or_peptide / ID: 2 / Number of copies: 26 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Salmonella typhimurium (bacteria) Salmonella typhimurium (bacteria) |
| Molecular weight | Theoretical: 24.726666 KDa |
| Sequence | String: MQKYALHAYP VMALMVATLT GCAWIPAKPL VQGATTAQPI PGPVPVANGS IFQSAQPINY GYQPLFEDRR PRNIGDTLTI VLQENVSAS KSSSANASRD GKTSFGFDTV PRYLQGLFGN SRADMEASGG NSFNGKGGAN ASNTFSGTLT VTVDQVLANG N LHVVGEKQ ...String: MQKYALHAYP VMALMVATLT GCAWIPAKPL VQGATTAQPI PGPVPVANGS IFQSAQPINY GYQPLFEDRR PRNIGDTLTI VLQENVSAS KSSSANASRD GKTSFGFDTV PRYLQGLFGN SRADMEASGG NSFNGKGGAN ASNTFSGTLT VTVDQVLANG N LHVVGEKQ IAINQGTEFI RFSGVVNPRT ISGSNSVPST QVADARIEYV GNGYINEAQN MGWLQRFFLN LSPM |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 Component:
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sugar embedding | Material: buffer | ||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: MOLYBDENUM / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 200 |
|---|---|
| Specialist optics | Energy filter - Name: In-column Omega Filter |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-50 / Number real images: 12759 / Average exposure time: 10.0 sec. / Average electron dose: 45.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated defocus max: 8.23 µm / Calibrated defocus min: 0.275 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 1.4 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 40000 |
| Sample stage | Specimen holder model: JEOL CRYOSPECPORTER / Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: OTHER / Overall B value: 38 |
|---|---|
| Output model |  PDB-7clr: |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)