[English] 日本語
 Yorodumi
Yorodumi- EMDB-19833: Human pseudouridine synthase 3 (PUS3 D118A mutant) and two pre-tR... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human pseudouridine synthase 3 (PUS3 D118A mutant) and two pre-tRNA-Arg | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | RNA modification / pseudouridylation / tRNA / homodimer / RNA BINDING PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationtRNA pseudouridine38/39 synthase / tRNA pseudouridine(38/39) synthase activity / mRNA pseudouridine synthesis / tRNA pseudouridine synthesis / pseudouridine synthase activity / tRNA modification in the nucleus and cytosol / tRNA modification / RNA binding / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
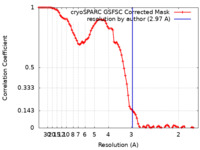
| Method | single particle reconstruction / cryo EM / Resolution: 2.97 Å | ||||||||||||
 Authors Authors | Lin T-Y / Jezowski J / Glatt S | ||||||||||||
| Funding support |  Poland, European Union, Poland, European Union,  Switzerland, 3 items Switzerland, 3 items
| ||||||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2024 Journal: Mol Cell / Year: 2024Title: The molecular basis of tRNA selectivity by human pseudouridine synthase 3. Authors: Ting-Yu Lin / Leon Kleemann / Jakub Jeżowski / Dominika Dobosz / Michał Rawski / Paulina Indyka / Grzegorz Ważny / Rahul Mehta / Andrzej Chramiec-Głąbik / Łukasz Koziej / Tristan Ranff ...Authors: Ting-Yu Lin / Leon Kleemann / Jakub Jeżowski / Dominika Dobosz / Michał Rawski / Paulina Indyka / Grzegorz Ważny / Rahul Mehta / Andrzej Chramiec-Głąbik / Łukasz Koziej / Tristan Ranff / Christian Fufezan / Mateusz Wawro / Jakub Kochan / Joanna Bereta / Sebastian A Leidel / Sebastian Glatt /    Abstract: Pseudouridine (Ψ), the isomer of uridine, is ubiquitously found in RNA, including tRNA, rRNA, and mRNA. Human pseudouridine synthase 3 (PUS3) catalyzes pseudouridylation of position 38/39 in tRNAs. ...Pseudouridine (Ψ), the isomer of uridine, is ubiquitously found in RNA, including tRNA, rRNA, and mRNA. Human pseudouridine synthase 3 (PUS3) catalyzes pseudouridylation of position 38/39 in tRNAs. However, the molecular mechanisms by which it recognizes its RNA targets and achieves site specificity remain elusive. Here, we determine single-particle cryo-EM structures of PUS3 in its apo form and bound to three tRNAs, showing how the symmetric PUS3 homodimer recognizes tRNAs and positions the target uridine next to its active site. Structure-guided and patient-derived mutations validate our structural findings in complementary biochemical assays. Furthermore, we deleted PUS1 and PUS3 in HEK293 cells and mapped transcriptome-wide Ψ sites by Pseudo-seq. Although PUS1-dependent sites were detectable in tRNA and mRNA, we found no evidence that human PUS3 modifies mRNAs. Our work provides the molecular basis for PUS3-mediated tRNA modification in humans and explains how its tRNA modification activity is linked to intellectual disabilities. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19833.map.gz emd_19833.map.gz | 31.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19833-v30.xml emd-19833-v30.xml emd-19833.xml emd-19833.xml | 19.7 KB 19.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19833_fsc.xml emd_19833_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_19833.png emd_19833.png | 92.8 KB | ||
| Filedesc metadata |  emd-19833.cif.gz emd-19833.cif.gz | 6.5 KB | ||
| Others |  emd_19833_half_map_1.map.gz emd_19833_half_map_1.map.gz emd_19833_half_map_2.map.gz emd_19833_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19833 http://ftp.pdbj.org/pub/emdb/structures/EMD-19833 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19833 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19833 | HTTPS FTP |
-Validation report
| Summary document |  emd_19833_validation.pdf.gz emd_19833_validation.pdf.gz | 794.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_19833_full_validation.pdf.gz emd_19833_full_validation.pdf.gz | 794 KB | Display | |
| Data in XML |  emd_19833_validation.xml.gz emd_19833_validation.xml.gz | 16.2 KB | Display | |
| Data in CIF |  emd_19833_validation.cif.gz emd_19833_validation.cif.gz | 21 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19833 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19833 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19833 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19833 | HTTPS FTP |
-Related structure data
| Related structure data |  9enfMC  8okdC  9enbC  9encC  9eneC  9f9qC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_19833.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19833.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_19833_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_19833_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Homodimer of human PUS3 and two pre-tRNA-Arg
| Entire | Name: Homodimer of human PUS3 and two pre-tRNA-Arg |
|---|---|
| Components |
|
-Supramolecule #1: Homodimer of human PUS3 and two pre-tRNA-Arg
| Supramolecule | Name: Homodimer of human PUS3 and two pre-tRNA-Arg / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Theoretical: 180 KDa |
-Supramolecule #2: Pseudouridine synthase 3 (PUS3)
| Supramolecule | Name: Pseudouridine synthase 3 (PUS3) / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: tRNA-Arg
| Supramolecule | Name: tRNA-Arg / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: tRNA pseudouridine(38/39) synthase
| Macromolecule | Name: tRNA pseudouridine(38/39) synthase / type: protein_or_peptide / ID: 1 / Details: D118A single mutation / Number of copies: 2 / Enantiomer: LEVO / EC number: tRNA pseudouridine38/39 synthase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 55.681207 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAYNDTDRNQ TEKLLKRVRE LEQEVQRLKK EQAKNKEDSN IRENSAGAGK TKRAFDFSAH GRRHVALRIA YMGWGYQGFA SQENTNNTI EEKLFEALTK TRLVESRQTS NYHRCGRTAK GVSAFGQVIS LDLRSQFPRG RDSEDFNVKE EANAAAEEIR Y THILNRVL ...String: MAYNDTDRNQ TEKLLKRVRE LEQEVQRLKK EQAKNKEDSN IRENSAGAGK TKRAFDFSAH GRRHVALRIA YMGWGYQGFA SQENTNNTI EEKLFEALTK TRLVESRQTS NYHRCGRTAK GVSAFGQVIS LDLRSQFPRG RDSEDFNVKE EANAAAEEIR Y THILNRVL PPDIRILAWA PVEPSFSARF SCLERTYRYF FPRADLDIVT MDYAAQKYVG THDFRNLCKM DVANGVINFQ RT ILSAQVQ LVGQSPGEGR WQEPFQLCQF EVTGQAFLYH QVRCMMAILF LIGQGMEKPE IIDELLNIEK NPQKPQYSMA VEF PLVLYD CKFENVKWIY DQEAQEFNIT HLQQLWANHA VKTHMLYSML QGLDTVPVPC GIGPKMDGMT EWGNVKPSVI KQTS AFVEG VKMRTYKPLM DRPKCQGLES RIQHFVRRGR IEHPHLFHEE ETKAKRDCND TLEEENTNLE TPTKRVCVDT EIKSI I UniProtKB: tRNA pseudouridine(38/39) synthase |
-Macromolecule #2: pre-tRNA-Arg
| Macromolecule | Name: pre-tRNA-Arg / type: rna / ID: 2 / Number of copies: 2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 30.2989 KDa |
| Sequence | String: GGCUCUGUGG CGCAAUGGAU AGCGCAUUGG ACUUCUAGCU GAGCCUAGUG UGGUCAUUCA AAGGUUGUGG GUUCGAGUCC CACCAGAGU CGCCA |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 8673 / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Refinement | Space: REAL / Protocol: OTHER |
| Output model |  PDB-9enf: |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)