[English] 日本語
 Yorodumi
Yorodumi- EMDB-18655: Cryo-EM reconstruction of VP5*/VP8* assembly from SA11 Rotavirus ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reconstruction of VP5*/VP8* assembly from SA11 Rotavirus Tripsinized Triple Layered Particle | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Rotavirus / dsRNA virus / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell rough endoplasmic reticulum / permeabilization of host organelle membrane involved in viral entry into host cell / host cytoskeleton / viral outer capsid / host cell endoplasmic reticulum-Golgi intermediate compartment / virion attachment to host cell / host cell plasma membrane Similarity search - Function | |||||||||
| Biological species |  Rotavirus / Rotavirus /  Rotavirus A Rotavirus A | |||||||||
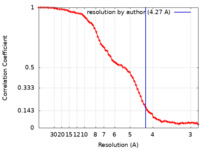
| Method | single particle reconstruction / cryo EM / Resolution: 4.27 Å | |||||||||
 Authors Authors | Asensio-Cob D / Perez-Mata C / Gomez-Blanco J / Vargas J / Rodriguez JM / Luque D | |||||||||
| Funding support |  Spain, 1 items Spain, 1 items
| |||||||||
 Citation Citation | Journal: bioRxiv / Year: 2025 Title: Structural determinants of rotavirus proteolytic activation. Authors: Dunia Asensio-Cob / Carlos P Mata / Josué Gómez-Blanco / Javier Vargas / Javier M Rodríguez / Daniel Luque /    Abstract: The infectivity of rotavirus (RV), the leading cause of childhood diarrhea, hinges on the activation of viral particles through the proteolysis of the spike protein by trypsin-like proteases in the ...The infectivity of rotavirus (RV), the leading cause of childhood diarrhea, hinges on the activation of viral particles through the proteolysis of the spike protein by trypsin-like proteases in the host intestinal lumen. Despite comprehensive structural characterization of the virus particle, the structural rationale behind the necessity of trypsin digestion of the VP4 protein for infectivity remains poorly understood. In this study, using cryo-electron microscopy (cryo-EM) and advanced image processing techniques, we compared uncleaved and cleaved RV virions and found that the conformation of the non-proteolyzed spike is constrained by the position of loops that surround its structure, linking the lectin domains of the spike head to its body. The proteolysis of these loops removes this structural constraint, thereby enabling the spike to undergo the necessary conformational changes required for cell membrane penetration. Thus, these loops function as regulatory elements to ensure that the spike protein is activated precisely when and where it is needed to facilitate a successful infection. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18655.map.gz emd_18655.map.gz | 665.8 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18655-v30.xml emd-18655-v30.xml emd-18655.xml emd-18655.xml | 20.3 KB 20.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_18655_fsc.xml emd_18655_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_18655.png emd_18655.png | 64.1 KB | ||
| Filedesc metadata |  emd-18655.cif.gz emd-18655.cif.gz | 6.5 KB | ||
| Others |  emd_18655_half_map_1.map.gz emd_18655_half_map_1.map.gz emd_18655_half_map_2.map.gz emd_18655_half_map_2.map.gz | 23.3 MB 23.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18655 http://ftp.pdbj.org/pub/emdb/structures/EMD-18655 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18655 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18655 | HTTPS FTP |
-Related structure data
| Related structure data |  8qtzMC  8olbC  8olcC  8oleC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_18655.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18655.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.43 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Rotavirus A
| Entire | Name:  Rotavirus A Rotavirus A |
|---|---|
| Components |
|
-Supramolecule #1: Rotavirus A
| Supramolecule | Name: Rotavirus A / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Rotavirus SA11 Non-tripsinized spike / NCBI-ID: 28875 / Sci species name: Rotavirus A / Sci species strain: SA11 / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Chlorocebus aethiops (grivet monkey) Chlorocebus aethiops (grivet monkey) |
| Molecular weight | Theoretical: 92 MDa |
| Virus shell | Shell ID: 1 / Name: TLP / Diameter: 700.0 Å |
-Macromolecule #1: Outer capsid protein VP4
| Macromolecule | Name: Outer capsid protein VP4 / type: protein_or_peptide / ID: 1 / Details: Outer capsid protein VP4 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Rotavirus Rotavirus |
| Molecular weight | Theoretical: 86.749891 KDa |
| Recombinant expression | Organism:  Chlorocebus aethiops (grivet monkey) Chlorocebus aethiops (grivet monkey) |
| Sequence | String: MASLIYRQLL TNSYTVDLSD EIQEIGSTKS QNVTINPGPF AQTGYAPVNW GPGEINDSTT VEPLLDGPYQ PTTFNPPVDY WMLLAPTTP GVIVEGTNNT DRWLATILIE PNVQSENRTY TIFGIQEQLT VSNTSQDQWK FIDVVKTTAN GSIGQYGSLL S SPKLYAVM ...String: MASLIYRQLL TNSYTVDLSD EIQEIGSTKS QNVTINPGPF AQTGYAPVNW GPGEINDSTT VEPLLDGPYQ PTTFNPPVDY WMLLAPTTP GVIVEGTNNT DRWLATILIE PNVQSENRTY TIFGIQEQLT VSNTSQDQWK FIDVVKTTAN GSIGQYGSLL S SPKLYAVM KHNEKLYTYE GQTPNARTGH YSTTNYDSVN MTAFCDFYII PRSEESKCTE YINNGLPPIQ NTRNVVPLSL TA RDVIHYR AQANEDIVIS KTSLWKEMQY NRDITIRFKF ANTIIKSGGL GYKWSEISFK PANYQYTYTR DGEEVTAHTT CSV NGVNDF SFNGGSLPTD FVVSKFEVIK ENSYVYIDYW DDSQAFRNVV YVRSLAANLN SVMCTGGSYN FSLPVGQWPV LTGG AVSLH SAGVTLSTQF TDFVSLNSLR FRFRLAVEEP HFKLTRTRLD RLYGLPAADP NNGKEYYEIA GRFSLISLVP SNDDY QTPI ANSVTVRQDL ERQLGELREE FNALSQEIAM SQLIDLALLP LDMFSMFSGI KSTIDAAKSM ATNVMKKFKK SGLANS VST LTDSLSDAAS SISRGSSIRS IGSSASAWTD VSTQITDISS SVSSVSTQTS TISRRLRLKE MATQTEGMNF DDISAAV LK TKIDKSTQIS PNTIPDIVTE ASEKFIPNRA YRVINNDDVF EAGIDGKFFA YKVDTFEEIP FDVQKFADLV TDSPVISA I IDFKTLKNLN DNYGITKQQA FNLLRSDPRV LREFINQDNP IIRNRIEQLI MQCRL UniProtKB: Outer capsid protein VP4 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 295 K / Instrument: LEICA EM GP |
| Details | Rotavirus SA11 Tripsinized spike |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Number real images: 2368 / Average electron dose: 39.9 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.75 µm / Nominal magnification: 58000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)