+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12576 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | ColicinE9 partial translocation complex | |||||||||
 Map data Map data | Output unsharpened map from relion refine3D job, sharpened using deepEMhancer tight target model | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | bacteriocin complex / import-stalled / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationcell cycle / extrachromosomal circular DNA / colicin transmembrane transporter activity / protein import / porin activity / pore complex / protein homotrimerization / monoatomic ion channel activity / monoatomic ion channel complex / cell outer membrane ...cell cycle / extrachromosomal circular DNA / colicin transmembrane transporter activity / protein import / porin activity / pore complex / protein homotrimerization / monoatomic ion channel activity / monoatomic ion channel complex / cell outer membrane / lipopolysaccharide binding / disordered domain specific binding / protein transport / monoatomic ion transmembrane transport / endonuclease activity / killing of cells of another organism / Hydrolases; Acting on ester bonds / periplasmic space / defense response to bacterium / protein domain specific binding / cell division / lipid binding / protein-containing complex / metal ion binding / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |    | |||||||||
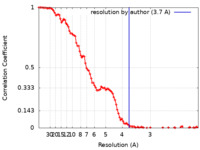
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Webby MN / Kleanthous C | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2021 Journal: EMBO J / Year: 2021Title: Porin threading drives receptor disengagement and establishes active colicin transport through Escherichia coli OmpF. Authors: Marie-Louise R Francis / Melissa N Webby / Nicholas G Housden / Renata Kaminska / Emma Elliston / Boonyaporn Chinthammit / Natalya Lukoyanova / Colin Kleanthous /  Abstract: Bacteria deploy weapons to kill their neighbours during competition for resources and to aid survival within microbiomes. Colicins were the first such antibacterial system identified, yet how these ...Bacteria deploy weapons to kill their neighbours during competition for resources and to aid survival within microbiomes. Colicins were the first such antibacterial system identified, yet how these bacteriocins cross the outer membrane (OM) of Escherichia coli is unknown. Here, by solving the structures of translocation intermediates via cryo-EM and by imaging toxin import, we uncover the mechanism by which the Tol-dependent nuclease colicin E9 (ColE9) crosses the bacterial OM. We show that threading of ColE9's disordered N-terminal domain through two pores of the trimeric porin OmpF causes the colicin to disengage from its primary receptor, BtuB, and reorganises the translocon either side of the membrane. Subsequent import of ColE9 through the lumen of a single OmpF subunit is driven by the proton-motive force, which is delivered by the TolQ-TolR-TolA-TolB assembly. Our study answers longstanding questions, such as why OmpF is a better translocator than OmpC, and reconciles the mechanisms by which both Tol- and Ton-dependent bacteriocins cross the bacterial outer membrane. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12576.map.gz emd_12576.map.gz | 74.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12576-v30.xml emd-12576-v30.xml emd-12576.xml emd-12576.xml | 22.7 KB 22.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12576_fsc.xml emd_12576_fsc.xml | 12.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_12576.png emd_12576.png | 13.2 KB | ||
| Filedesc metadata |  emd-12576.cif.gz emd-12576.cif.gz | 6.9 KB | ||
| Others |  emd_12576_additional_1.map.gz emd_12576_additional_1.map.gz emd_12576_half_map_1.map.gz emd_12576_half_map_1.map.gz emd_12576_half_map_2.map.gz emd_12576_half_map_2.map.gz | 65.1 MB 65.3 MB 65.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12576 http://ftp.pdbj.org/pub/emdb/structures/EMD-12576 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12576 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12576 | HTTPS FTP |
-Related structure data
| Related structure data |  7nstMC  7nsuC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12576.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12576.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Output unsharpened map from relion refine3D job, sharpened using deepEMhancer tight target model | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.047 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Unsharpened map from relion refine3D job
| File | emd_12576_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map from relion refine3D job | ||||||||||||
| Projections & Slices |
| ||||||||||||
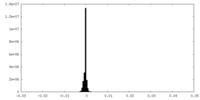
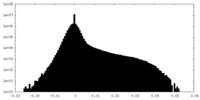
| Density Histograms |
-Half map: half map A from refine3D in relion3.1
| File | emd_12576_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A from refine3D in relion3.1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
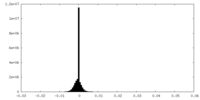
| Density Histograms |
-Half map: half map B from refine3D in relion3.1
| File | emd_12576_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B from refine3D in relion3.1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
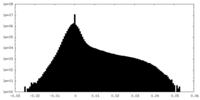
| Density Histograms |
- Sample components
Sample components
-Entire : ColicinE9 intact translocon complex
| Entire | Name: ColicinE9 intact translocon complex |
|---|---|
| Components |
|
-Supramolecule #1: ColicinE9 intact translocon complex
| Supramolecule | Name: ColicinE9 intact translocon complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Bacteriocin colicinE9 bound to outer membrane protein receptor BtuB and translocator ompF. Disulphide linked to tolB. |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 256 KDa |
-Macromolecule #1: Outer membrane protein F
| Macromolecule | Name: Outer membrane protein F / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 37.11425 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: AEIYNKDGNK VDLYGKAVGL HYFSKGNGEN SYGGNGDMTY ARLGFKGETQ INSDLTGYGQ WEYNFQGNNS EGADAQTGNK TRLAFAGLK YADVGSFDYG RNYGVVYDAL GYTDMLPEFG GDTAYSDDFF VGRVGGVATY RNSNFFGLVD GLNFAVQYLG K NERDTARR ...String: AEIYNKDGNK VDLYGKAVGL HYFSKGNGEN SYGGNGDMTY ARLGFKGETQ INSDLTGYGQ WEYNFQGNNS EGADAQTGNK TRLAFAGLK YADVGSFDYG RNYGVVYDAL GYTDMLPEFG GDTAYSDDFF VGRVGGVATY RNSNFFGLVD GLNFAVQYLG K NERDTARR SNGDGVGGSI SYEYEGFGIV GAYGAADRTN LQEAQPLGNG KKAEQWATGL KYDANNIYLA ANYGETRNAT PI TNKFTNT SGFANKTQDV LLVAQYQFDF GLRPSIAYTK SKAKDVEGIG DVDLVNYFEV GATYYFNKNM STYVDYIINQ IDS DNKLGV GSDDTVAVGI VYQF UniProtKB: Outer membrane porin F |
-Macromolecule #2: Colicin-E9
| Macromolecule | Name: Colicin-E9 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 31.563652 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGGDGRGHN TGAHSTSGNI NGGPTGIGVS GGCSDGSGWS SENNPWGGGS GSGIHWGGGS GRGNGGGNGN SGGGSGTGGN LSAVAAPVA FGFPALSTPG AGGLAVSISA SELSAAIAGI IAKLKKVNLK FTPFGVVLSS LIPSEIAKDD PNMMSKIVTS L PADDITES ...String: MSGGDGRGHN TGAHSTSGNI NGGPTGIGVS GGCSDGSGWS SENNPWGGGS GSGIHWGGGS GRGNGGGNGN SGGGSGTGGN LSAVAAPVA FGFPALSTPG AGGLAVSISA SELSAAIAGI IAKLKKVNLK FTPFGVVLSS LIPSEIAKDD PNMMSKIVTS L PADDITES PVSSLPLDKA TVNVNVRVVD DVKDERQNIS VVSGVPMSVP VVDAKPTERP GVFTASIPGA PVLNISVNDS TP AVQTLSP GVTNNTDKDV RPAGFTQGGN TRDAVIRFPK DSGHNAVYVS VSDVLSPDQV KQRQDEENRR QQEWDAT UniProtKB: Colicin-E9 |
-Macromolecule #3: Tol-Pal system protein TolB
| Macromolecule | Name: Tol-Pal system protein TolB / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 46.000285 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKQALRVAFG FLILWASVLH AEVRIVIDSG VDSGRPIGVV PFQWAGPGAA PEDIGGIVAA DLRNSGKFNP LDRARLPQQP GSAQEVQPA AWSALGIDAV VVGQVTPNPD GSYNVAYQLV DTGGAPGTVL AQNSYKVNKQ WLRYAGHTAS DEVFEKLTGI K GAFRTRIA ...String: MKQALRVAFG FLILWASVLH AEVRIVIDSG VDSGRPIGVV PFQWAGPGAA PEDIGGIVAA DLRNSGKFNP LDRARLPQQP GSAQEVQPA AWSALGIDAV VVGQVTPNPD GSYNVAYQLV DTGGAPGTVL AQNSYKVNKQ WLRYAGHTAS DEVFEKLTGI K GAFRTRIA YVVQTNGGQF PYELRVSDYD GYNQFVVHRS PQCLMSPAWS PDGSKLAYVT FESGRSALVI QTLANGAVRQ VA SFPRHNG APAFSPDGSK LAFALSKTGS LNLYVMDLAS GQIRQVTDGR SNNTEPTWFP DSQNLAFTSD QAGRPQVYKV NIN GGAPQR ITWEGSQNQD ADVSSDGKFM VMVSSNGGQQ HIAKQDLATG GVQVLSSTFL DETPSLAPNG TMVIYSSSQG MGSV LNLVS TDGRFKARLP ATDGQVKFPA WSPYL UniProtKB: Tol-Pal system protein TolB |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.9 |
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: GRAPHENE OXIDE / Support film - #0 - topology: CONTINUOUS / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: CARBON / Support film - #1 - topology: HOLEY / Support film - #1 - Film thickness: 20 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Details: PELCO easiGlow, Ted Pella Inc, USA |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 92 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV Details: 3 uL of diluted translocon preparation were applied on freshly glow discharged grids coated with graphene oxide (as described in https://doi.org/10.1038/s41594-019-0355-2); after 30sec ...Details: 3 uL of diluted translocon preparation were applied on freshly glow discharged grids coated with graphene oxide (as described in https://doi.org/10.1038/s41594-019-0355-2); after 30sec waiting grids were blotted for 8-10 using -10 force and plunge frozen in liquid ethane. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Number grids imaged: 2 / Number real images: 3000 / Average exposure time: 10.0 sec. / Average electron dose: 49.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated defocus max: 3.8000000000000003 µm / Calibrated defocus min: 1.23 µm / Calibrated magnification: 47755 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 47619 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)