+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0834 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
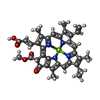
| Title | Structure of the peripheral FCPI from diatom | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |  Chaetoceros gracilis (Diatom) Chaetoceros gracilis (Diatom) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.6 Å | |||||||||
 Authors Authors | Nagao R / Kato K / Miyazaki N / Akita F / Shen JR | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Structural basis for assembly and function of a diatom photosystem I-light-harvesting supercomplex. Authors: Ryo Nagao / Koji Kato / Kentaro Ifuku / Takehiro Suzuki / Minoru Kumazawa / Ikuo Uchiyama / Yasuhiro Kashino / Naoshi Dohmae / Seiji Akimoto / Jian-Ren Shen / Naoyuki Miyazaki / Fusamichi Akita /  Abstract: Photosynthetic light-harvesting complexes (LHCs) play a pivotal role in collecting solar energy for photochemical reactions in photosynthesis. One of the major LHCs are fucoxanthin chlorophyll a/c- ...Photosynthetic light-harvesting complexes (LHCs) play a pivotal role in collecting solar energy for photochemical reactions in photosynthesis. One of the major LHCs are fucoxanthin chlorophyll a/c-binding proteins (FCPs) present in diatoms, a group of organisms having important contribution to the global carbon cycle. Here, we report a 2.40-Å resolution structure of the diatom photosystem I (PSI)-FCPI supercomplex by cryo-electron microscopy. The supercomplex is composed of 16 different FCPI subunits surrounding a monomeric PSI core. Each FCPI subunit showed different protein structures with different pigment contents and binding sites, and they form a complicated pigment-protein network together with the PSI core to harvest and transfer the light energy efficiently. In addition, two unique, previously unidentified subunits were found in the PSI core. The structure provides numerous insights into not only the light-harvesting strategy in diatom PSI-FCPI but also evolutionary dynamics of light harvesters among oxyphototrophs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0834.map.gz emd_0834.map.gz | 28.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0834-v30.xml emd-0834-v30.xml emd-0834.xml emd-0834.xml | 23.8 KB 23.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0834_fsc.xml emd_0834_fsc.xml | 17.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_0834.png emd_0834.png | 77.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0834 http://ftp.pdbj.org/pub/emdb/structures/EMD-0834 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0834 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0834 | HTTPS FTP |
-Validation report
| Summary document |  emd_0834_validation.pdf.gz emd_0834_validation.pdf.gz | 344.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_0834_full_validation.pdf.gz emd_0834_full_validation.pdf.gz | 343.9 KB | Display | |
| Data in XML |  emd_0834_validation.xml.gz emd_0834_validation.xml.gz | 15.9 KB | Display | |
| Data in CIF |  emd_0834_validation.cif.gz emd_0834_validation.cif.gz | 22.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0834 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0834 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0834 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0834 | HTTPS FTP |
-Related structure data
| Related structure data |  6l4tMC  0835C  6l4uC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_0834.map.gz / Format: CCP4 / Size: 488.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0834.map.gz / Format: CCP4 / Size: 488.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.113 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : peripheral FCPI
+Supramolecule #1: peripheral FCPI
+Macromolecule #1: Fucoxanthin chlorophyll a/c-binding protein Lhcr12
+Macromolecule #2: Fucoxanthin chlorophyll a/c-binding protein Lhcr10
+Macromolecule #3: Fucoxanthin chlorophyll a/c-binding protein Lhcr4
+Macromolecule #4: Fucoxanthin chlorophyll a/c-binding protein Lhcr3
+Macromolecule #5: Fucoxanthin chlorophyll a/c-binding protein Lhcq13
+Macromolecule #6: Fucoxanthin chlorophyll a/c-binding protein Lhcq3
+Macromolecule #7: Fucoxanthin chlorophyll a/c-binding protein Lhcq11
+Macromolecule #8: Fucoxanthin chlorophyll a/c-binding protein Lhcq10
+Macromolecule #9: Fucoxanthin chlorophyll a/c-binding protein Lhcq8
+Macromolecule #10: Fucoxanthin chlorophyll a/c-binding protein Lhcq5
+Macromolecule #11: CHLOROPHYLL A
+Macromolecule #12: Chlorophyll c1
+Macromolecule #13: (3S,3'R,5R,6S,7cis)-7',8'-didehydro-5,6-dihydro-5,6-epoxy-beta,be...
+Macromolecule #14: (3S,3'S,5R,5'R,6S,6'R,8'R)-3,5'-dihydroxy-8-oxo-6',7'-didehydro-5...
+Macromolecule #15: 1,2-DIPALMITOYL-PHOSPHATIDYL-GLYCEROLE
+Macromolecule #16: 1,2-DISTEAROYL-MONOGALACTOSYL-DIGLYCERIDE
+Macromolecule #17: DODECYL-BETA-D-MALTOSIDE
+Macromolecule #18: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 6.5 Component:
| |||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: MOLYBDENUM / Mesh: 300 / Support film - Material: CARBON / Pretreatment - Type: PLASMA CLEANING | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-6l4t: |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)