Yorodumi
Yorodumi+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4844 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Polytomella F-ATP synthase, Rotary substate 2C, focussed refinement of F1 head and rotor | |||||||||
 Map data Map data | None | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | mitochondrial ATP synthase dimer flexible coupling cryoEM / PROTON TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology informationproton transmembrane transporter activity / proton motive force-driven ATP synthesis / proton-transporting two-sector ATPase complex, proton-transporting domain / proton motive force-driven mitochondrial ATP synthesis / H+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / ADP binding / mitochondrial inner membrane / hydrolase activity ...proton transmembrane transporter activity / proton motive force-driven ATP synthesis / proton-transporting two-sector ATPase complex, proton-transporting domain / proton motive force-driven mitochondrial ATP synthesis / H+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / ADP binding / mitochondrial inner membrane / hydrolase activity / lipid binding / mitochondrion / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |  Polytomella sp. Pringsheim 198.80 (plant) Polytomella sp. Pringsheim 198.80 (plant) | |||||||||
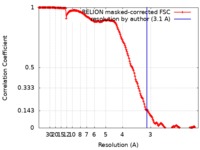
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Murphy BJ / Klusch N | |||||||||
| Funding support |  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2019 Journal: Science / Year: 2019Title: Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F-F coupling. Authors: Bonnie J Murphy / Niklas Klusch / Julian Langer / Deryck J Mills / Özkan Yildiz / Werner Kühlbrandt /  Abstract: FF-adenosine triphosphate (ATP) synthases make the energy of the proton-motive force available for energy-consuming processes in the cell. We determined the single-particle cryo-electron microscopy ...FF-adenosine triphosphate (ATP) synthases make the energy of the proton-motive force available for energy-consuming processes in the cell. We determined the single-particle cryo-electron microscopy structure of active dimeric ATP synthase from mitochondria of sp. at a resolution of 2.7 to 2.8 angstroms. Separation of 13 well-defined rotary substates by three-dimensional classification provides a detailed picture of the molecular motions that accompany -ring rotation and result in ATP synthesis. Crucially, the F head rotates along with the central stalk and -ring rotor for the first ~30° of each 120° primary rotary step to facilitate flexible coupling of the stoichiometrically mismatched F and F subcomplexes. Flexibility is mediated primarily by the interdomain hinge of the conserved OSCP subunit. A conserved metal ion in the proton access channel may synchronize -ring protonation with rotation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4844.map.gz emd_4844.map.gz | 395.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4844-v30.xml emd-4844-v30.xml emd-4844.xml emd-4844.xml | 23.5 KB 23.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4844_fsc.xml emd_4844_fsc.xml | 17 KB | Display |  FSC data file FSC data file |
| Images |  emd_4844.png emd_4844.png | 175.5 KB | ||
| Filedesc metadata |  emd-4844.cif.gz emd-4844.cif.gz | 7.3 KB | ||
| Others |  emd_4844_half_map_1.map.gz emd_4844_half_map_1.map.gz emd_4844_half_map_2.map.gz emd_4844_half_map_2.map.gz | 337.6 MB 337.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4844 http://ftp.pdbj.org/pub/emdb/structures/EMD-4844 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4844 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4844 | HTTPS FTP |
-Related structure data
| Related structure data |  6re7MC  4805C  4806C  4807C  4808C  4809C  4810C  4811C  4812C  4813C  4814C  4815C  4816C  4817C  4818C  4819C  4820C  4821C  4822C  4823C  4824C  4825C  4826C  4827C  4828C  4829C  4830C  4831C  4832C  4833C  4834C  4835C  4836C  4837C  4838C  4839C  4840C  4841C  4842C  4843C  4845C  4846C  4847C  4848C  4849C  4850C  4851C  4852C  4853C  4854C  4855C  4856C  4857C  6rd4C  6rd5C  6rd6C  6rd7C  6rd8C  6rd9C  6rdaC  6rdbC  6rdcC  6rddC  6rdeC  6rdfC  6rdgC  6rdhC  6rdiC  6rdjC  6rdkC  6rdlC  6rdmC  6rdnC  6rdoC  6rdpC  6rdqC  6rdrC  6rdsC  6rdtC  6rduC  6rdvC  6rdwC  6rdxC  6rdyC  6rdzC  6re0C  6re1C  6re2C  6re3C  6re4C  6re5C  6re6C  6re8C  6re9C  6reaC  6rebC  6recC  6redC  6reeC  6refC  6repC  6rerC  6resC  6retC  6reuC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10375 (Title: Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F1-Fo coupling EMPIAR-10375 (Title: Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F1-Fo couplingData size: 43.8 TB Data #1: Unaligned frames, gain reference corrected [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4844.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4844.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.053 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: None
| File | emd_4844_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||
| Projections & Slices |
| ||||||||||||
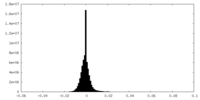
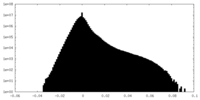
| Density Histograms |
-Half map: None
| File | emd_4844_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||
| Projections & Slices |
| ||||||||||||
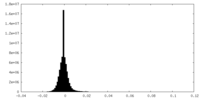
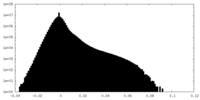
| Density Histograms |
- Sample components
Sample components
+Entire : Mitochondrial F-ATP synthase dimer from Polytomella sp. Pringshei...
+Supramolecule #1: Mitochondrial F-ATP synthase dimer from Polytomella sp. Pringshei...
+Macromolecule #1: Mitochondrial ATP synthase subunit c
+Macromolecule #2: Mitochondrial ATP synthase subunit OSCP
+Macromolecule #3: epsilon: Polytomella F-ATP synthase epsilon subunit
+Macromolecule #4: Mitochondrial ATP synthase subunit delta
+Macromolecule #5: ATP synthase gamma chain, mitochondrial
+Macromolecule #6: ATP synthase subunit alpha
+Macromolecule #7: ATP synthase subunit beta
+Macromolecule #8: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #9: MAGNESIUM ION
+Macromolecule #10: ADENOSINE-5'-DIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.5 mg/mL |
|---|---|
| Buffer | pH: 7.8 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3840 pixel / Digitization - Dimensions - Height: 3712 pixel / Average electron dose: 35.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -5.0 µm / Nominal defocus min: -0.4 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-6re7: |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)