+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22079 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | EM Structure of Full-Length Androgen Receptor and DNA Complex | |||||||||
 Map data Map data | AR/ARE-DNA complex. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 13.0 Å | |||||||||
 Authors Authors | Yu X / Yi P | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2020 Journal: Mol Cell / Year: 2020Title: Structural Insights of Transcriptionally Active, Full-Length Androgen Receptor Coactivator Complexes. Authors: Xinzhe Yu / Ping Yi / Ross A Hamilton / Hong Shen / Muyuan Chen / Charles E Foulds / Michael A Mancini / Steven J Ludtke / Zhao Wang / Bert W O'Malley /  Abstract: Steroid receptors activate gene transcription by recruiting coactivators to initiate transcription of their target genes. For most nuclear receptors, the ligand-dependent activation function domain-2 ...Steroid receptors activate gene transcription by recruiting coactivators to initiate transcription of their target genes. For most nuclear receptors, the ligand-dependent activation function domain-2 (AF-2) is a primary contributor to the nuclear receptor (NR) transcriptional activity. In contrast to other steroid receptors, such as ERα, the activation function of androgen receptor (AR) is largely dependent on its ligand-independent AF-1 located in its N-terminal domain (NTD). It remains unclear why AR utilizes a different AF domain from other receptors despite that NRs share similar domain organizations. Here, we present cryoelectron microscopy (cryo-EM) structures of DNA-bound full-length AR and its complex structure with key coactivators, SRC-3 and p300. AR dimerization follows a unique head-to-head and tail-to-tail manner. Unlike ERα, AR directly contacts a single SRC-3 and p300. The AR NTD is the primary site for coactivator recruitment. The structures provide a basis for understanding assembly of the AR:coactivator complex and its domain contributions for coactivator assembly and transcriptional regulation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22079.map.gz emd_22079.map.gz | 27.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22079-v30.xml emd-22079-v30.xml emd-22079.xml emd-22079.xml | 15.3 KB 15.3 KB | Display Display |  EMDB header EMDB header |
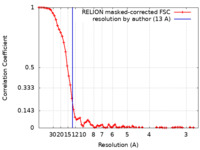
| FSC (resolution estimation) |  emd_22079_fsc.xml emd_22079_fsc.xml | 7.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_22079.png emd_22079.png | 78.2 KB | ||
| Others |  emd_22079_additional_1.map.gz emd_22079_additional_1.map.gz emd_22079_additional_2.map.gz emd_22079_additional_2.map.gz | 7 MB 6.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22079 http://ftp.pdbj.org/pub/emdb/structures/EMD-22079 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22079 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22079 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10435 (Title: Structural Insights of Transcriptionally Active, Full-Length Androgen Receptor Coactivator Complexes EMPIAR-10435 (Title: Structural Insights of Transcriptionally Active, Full-Length Androgen Receptor Coactivator ComplexesData size: 5.1 TB / Data #1: ARE-DNA/AR [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_22079.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22079.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | AR/ARE-DNA complex. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.39 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
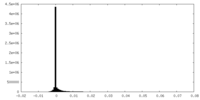
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: AR/ARE-DNA complex with one AR-Ab2 antibody binding. AR-Ab2...
| File | emd_22079_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | AR/ARE-DNA complex with one AR-Ab2 antibody binding. AR-Ab2 recognizes the region adjacent to the FXXLF motif (residues 23-27). | ||||||||||||
| Projections & Slices |
| ||||||||||||
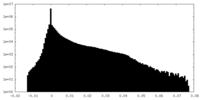
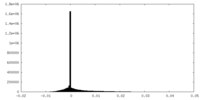
| Density Histograms |
-Additional map: AR/ARE-DNA complex with two AR-Ab1 antibodies binding. AR-Ab1...
| File | emd_22079_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | AR/ARE-DNA complex with two AR-Ab1 antibodies binding. AR-Ab1 recognizes AR NTD (residues 98-503). One Ab1 density is much stronger than the other one. | ||||||||||||
| Projections & Slices |
| ||||||||||||
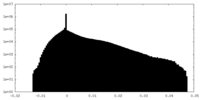
| Density Histograms |
- Sample components
Sample components
-Entire : AR/ARE-DNA complex
| Entire | Name: AR/ARE-DNA complex |
|---|---|
| Components |
|
-Supramolecule #1: AR/ARE-DNA complex
| Supramolecule | Name: AR/ARE-DNA complex / type: complex / ID: 1 / Parent: 0 / Details: Androgen Receptor and DNA complex |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism: Insect cell expression vector pTIE1 (others) |
| Molecular weight | Experimental: 370 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.01 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Chamber temperature: 291 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Number real images: 1958 / Average exposure time: 10.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)