[English] 日本語
 Yorodumi
Yorodumi- EMDB-12853: Icosahedral cryo-EM reconstruction of Haliangium ochraceum encapsulin -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12853 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Icosahedral cryo-EM reconstruction of Haliangium ochraceum encapsulin | |||||||||
 Map data Map data | Refine3D map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Encapsulin / encapsulated ferritin / haliangium ochraceum / VIRUS LIKE PARTICLE | |||||||||
| Function / homology | Type 1 encapsulin shell protein / Encapsulating protein for peroxidase / : / encapsulin nanocompartment / iron ion transport / intracellular iron ion homeostasis / Type 1 encapsulin shell protein Function and homology information Function and homology information | |||||||||
| Biological species |  Haliangium ochraceum (bacteria) / Haliangium ochraceum (bacteria) /  Haliangium ochraceum (strain DSM 14365 / JCM 11303 / SMP-2) (bacteria) Haliangium ochraceum (strain DSM 14365 / JCM 11303 / SMP-2) (bacteria) | |||||||||
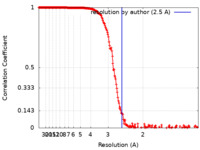
| Method | single particle reconstruction / cryo EM / Resolution: 2.5 Å | |||||||||
 Authors Authors | Marles-Wright J / Basle A | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2022 Journal: Sci Adv / Year: 2022Title: Pore dynamics and asymmetric cargo loading in an encapsulin nanocompartment. Authors: Jennifer Ross / Zak McIver / Thomas Lambert / Cecilia Piergentili / Jasmine Emma Bird / Kelly J Gallagher / Faye L Cruickshank / Patrick James / Efrain Zarazúa-Arvizu / Louise E Horsfall / ...Authors: Jennifer Ross / Zak McIver / Thomas Lambert / Cecilia Piergentili / Jasmine Emma Bird / Kelly J Gallagher / Faye L Cruickshank / Patrick James / Efrain Zarazúa-Arvizu / Louise E Horsfall / Kevin J Waldron / Marcus D Wilson / C Logan Mackay / Arnaud Baslé / David J Clarke / Jon Marles-Wright /  Abstract: Encapsulins are protein nanocompartments that house various cargo enzymes, including a family of decameric ferritin-like proteins. Here, we study a recombinant encapsulin:encapsulated ferritin ...Encapsulins are protein nanocompartments that house various cargo enzymes, including a family of decameric ferritin-like proteins. Here, we study a recombinant encapsulin:encapsulated ferritin complex using cryo-electron microscopy and hydrogen/deuterium exchange mass spectrometry to gain insight into the structural relationship between the encapsulin shell and its protein cargo. An asymmetric single-particle reconstruction reveals four encapsulated ferritin decamers in a tetrahedral arrangement within the encapsulin nanocompartment. This leads to a symmetry mismatch between the protein cargo and the icosahedral encapsulin shell. The encapsulated ferritin decamers are offset from the interior face of the encapsulin shell. Using hydrogen/deuterium exchange mass spectrometry, we observed the dynamic behavior of the major fivefold pore in the encapsulin shell and show the pore opening via the movement of the encapsulin A-domain. These data will accelerate efforts to engineer the encapsulation of heterologous cargo proteins and to alter the permeability of the encapsulin shell via pore modifications. #1:  Journal: Biorxiv / Year: 2021 Journal: Biorxiv / Year: 2021Title: Model of Haliangium ochraceum encapsulin from icosahedral single particle reconstruction Authors: Marles-Wright J / Basle A / Clarke DJ / Ross J | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12853.map.gz emd_12853.map.gz | 567.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12853-v30.xml emd-12853-v30.xml emd-12853.xml emd-12853.xml | 22.5 KB 22.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12853_fsc.xml emd_12853_fsc.xml | 26.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_12853.png emd_12853.png | 91.2 KB | ||
| Filedesc metadata |  emd-12853.cif.gz emd-12853.cif.gz | 6.5 KB | ||
| Others |  emd_12853_additional_1.map.gz emd_12853_additional_1.map.gz emd_12853_half_map_1.map.gz emd_12853_half_map_1.map.gz emd_12853_half_map_2.map.gz emd_12853_half_map_2.map.gz | 2.8 MB 582.4 MB 582.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12853 http://ftp.pdbj.org/pub/emdb/structures/EMD-12853 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12853 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12853 | HTTPS FTP |
-Related structure data
| Related structure data |  7odwMC  7oe2C  7oeuC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10716 (Title: Cryo electron microscopy of single particles of the Haliangium ochraceum Encapsulin:encapsulated ferritin encapsulin nano compartment EMPIAR-10716 (Title: Cryo electron microscopy of single particles of the Haliangium ochraceum Encapsulin:encapsulated ferritin encapsulin nano compartmentData size: 1.7 TB Data #1: Multi-frame micrographs and particle sets for Haliangium ochraceum encapsulin:encapsulated ferritin reconstruction [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12853.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12853.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refine3D map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.652 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
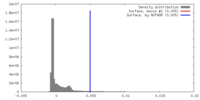
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
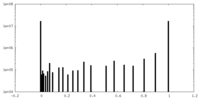
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Solvent Mask
| File | emd_12853_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Solvent Mask | ||||||||||||
| Projections & Slices |
| ||||||||||||
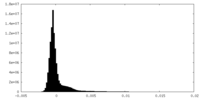
| Density Histograms |
-Half map: Half map 1
| File | emd_12853_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
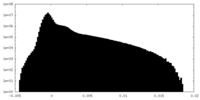
| Density Histograms |
-Half map: Half map 2
| File | emd_12853_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Ternary complex of Haliangium ochraceum encapsulin and encapsulat...
| Entire | Name: Ternary complex of Haliangium ochraceum encapsulin and encapsulated ferritin proteins |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of Haliangium ochraceum encapsulin and encapsulat...
| Supramolecule | Name: Ternary complex of Haliangium ochraceum encapsulin and encapsulated ferritin proteins type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Complex produced by co-expression in E. coli |
|---|---|
| Source (natural) | Organism:  Haliangium ochraceum (bacteria) Haliangium ochraceum (bacteria) |
| Molecular weight | Theoretical: 1.8 MDa |
-Macromolecule #1: Linocin_M18 bacteriocin protein
| Macromolecule | Name: Linocin_M18 bacteriocin protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Haliangium ochraceum (strain DSM 14365 / JCM 11303 / SMP-2) (bacteria) Haliangium ochraceum (strain DSM 14365 / JCM 11303 / SMP-2) (bacteria)Strain: DSM 14365 / JCM 11303 / SMP-2 |
| Molecular weight | Theoretical: 28.844598 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDLLKRHLAP IVPDAWSAID EEAKEIFQGH LAGRKLVDFR GPFGWEYAAV NTGELRPIDD TPEDVDMKLR QVQPLAEVRV PFTLDVTEL DSVARGATNP DLDDVARAAE RMVEAEDSAI FHGWAQAGIK GIVDSTPHEA LAVASVSDFP RAVLSAADTL R KAGVTGPY ...String: MDLLKRHLAP IVPDAWSAID EEAKEIFQGH LAGRKLVDFR GPFGWEYAAV NTGELRPIDD TPEDVDMKLR QVQPLAEVRV PFTLDVTEL DSVARGATNP DLDDVARAAE RMVEAEDSAI FHGWAQAGIK GIVDSTPHEA LAVASVSDFP RAVLSAADTL R KAGVTGPY ALVLGPKAYD DLFAATQDGY PVAKQVQRLV VDGPLVRANA LAGALVMSMR GGDYELTVGQ DLSIGYAFHD RS KVELFVA ESFTFRVLEP GAAVHLRYA UniProtKB: Type 1 encapsulin shell protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: Solutions were prepared with MilliQ water and filtered using a 0.22 um filter. | |||||||||
| Grid | Model: Quantifoil / Material: GOLD / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV Details: blot force -5, wait time 10 seconds and blot time of 3 seconds. | |||||||||
| Details | Sample mono disperse as determined by SEC |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 11520 pixel / Digitization - Dimensions - Height: 8184 pixel / Number grids imaged: 1 / Number real images: 8109 / Average exposure time: 1.0 sec. / Average electron dose: 40.509 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | Initial fitting performed using chimera with real space refinement in Phenix. |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Overall B value: 86 / Target criteria: CC |
| Output model |  PDB-7odw: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)