[English] 日本語
 Yorodumi
Yorodumi- EMDB-13608: Focused reconstruction of Haliangium ochraceum encapsulated ferri... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Focused reconstruction of Haliangium ochraceum encapsulated ferritin cargo within the encapsulin nano compartment | |||||||||
 Map data Map data | CryroSPARC map | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Haliangium ochraceum (bacteria) Haliangium ochraceum (bacteria) | |||||||||
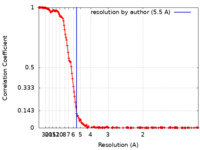
| Method | single particle reconstruction / cryo EM / Resolution: 5.5 Å | |||||||||
 Authors Authors | Marles-Wright J / Basle A / Ross J / Clarke DJ | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2022 Journal: Sci Adv / Year: 2022Title: Pore dynamics and asymmetric cargo loading in an encapsulin nanocompartment. Authors: Jennifer Ross / Zak McIver / Thomas Lambert / Cecilia Piergentili / Jasmine Emma Bird / Kelly J Gallagher / Faye L Cruickshank / Patrick James / Efrain Zarazúa-Arvizu / Louise E Horsfall / ...Authors: Jennifer Ross / Zak McIver / Thomas Lambert / Cecilia Piergentili / Jasmine Emma Bird / Kelly J Gallagher / Faye L Cruickshank / Patrick James / Efrain Zarazúa-Arvizu / Louise E Horsfall / Kevin J Waldron / Marcus D Wilson / C Logan Mackay / Arnaud Baslé / David J Clarke / Jon Marles-Wright /  Abstract: Encapsulins are protein nanocompartments that house various cargo enzymes, including a family of decameric ferritin-like proteins. Here, we study a recombinant encapsulin:encapsulated ferritin ...Encapsulins are protein nanocompartments that house various cargo enzymes, including a family of decameric ferritin-like proteins. Here, we study a recombinant encapsulin:encapsulated ferritin complex using cryo-electron microscopy and hydrogen/deuterium exchange mass spectrometry to gain insight into the structural relationship between the encapsulin shell and its protein cargo. An asymmetric single-particle reconstruction reveals four encapsulated ferritin decamers in a tetrahedral arrangement within the encapsulin nanocompartment. This leads to a symmetry mismatch between the protein cargo and the icosahedral encapsulin shell. The encapsulated ferritin decamers are offset from the interior face of the encapsulin shell. Using hydrogen/deuterium exchange mass spectrometry, we observed the dynamic behavior of the major fivefold pore in the encapsulin shell and show the pore opening via the movement of the encapsulin A-domain. These data will accelerate efforts to engineer the encapsulation of heterologous cargo proteins and to alter the permeability of the encapsulin shell via pore modifications. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13608.map.gz emd_13608.map.gz | 351.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13608-v30.xml emd-13608-v30.xml emd-13608.xml emd-13608.xml | 22.9 KB 22.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13608_fsc.xml emd_13608_fsc.xml | 22.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_13608.png emd_13608.png | 44.7 KB | ||
| Masks |  emd_13608_msk_1.map emd_13608_msk_1.map | 421.9 MB |  Mask map Mask map | |
| Others |  emd_13608_additional_1.map.gz emd_13608_additional_1.map.gz emd_13608_half_map_1.map.gz emd_13608_half_map_1.map.gz emd_13608_half_map_2.map.gz emd_13608_half_map_2.map.gz | 393.4 MB 389.3 MB 389.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13608 http://ftp.pdbj.org/pub/emdb/structures/EMD-13608 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13608 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13608 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_13608.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13608.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryroSPARC map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.652 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_13608_msk_1.map emd_13608_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
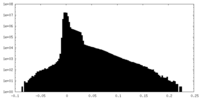
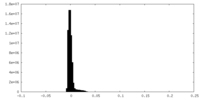
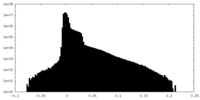
| Density Histograms |
-Additional map: Sharpened map
| File | emd_13608_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_13608_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B
| File | emd_13608_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Ternary complex of Haliangium ochraceum encapsulin and encapsulat...
| Entire | Name: Ternary complex of Haliangium ochraceum encapsulin and encapsulated ferritin proteins |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of Haliangium ochraceum encapsulin and encapsulat...
| Supramolecule | Name: Ternary complex of Haliangium ochraceum encapsulin and encapsulated ferritin proteins type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Complex produced by co-expression in E. coli |
|---|---|
| Source (natural) | Organism:  Haliangium ochraceum (bacteria) Haliangium ochraceum (bacteria) |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 1.8 MDa |
-Supramolecule #2: Encapsulin nano compartment
| Supramolecule | Name: Encapsulin nano compartment / type: complex / ID: 2 / Parent: 1 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Haliangium ochraceum (bacteria) Haliangium ochraceum (bacteria) |
| Recombinant expression | Organism:  |
-Macromolecule #1: Haliangium ochraceum encapsulin
| Macromolecule | Name: Haliangium ochraceum encapsulin / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Haliangium ochraceum (bacteria) Haliangium ochraceum (bacteria) |
| Recombinant expression | Organism:  |
| Sequence | String: MDLLKRHLAP IVPDAWSAID EEAKEIFQGH LAGRKLVDFR GPFGWEYAAV NTGELRPIDD TPEDVDMKL RQVQPLAEVR VPFTLDVTEL DSVARGATNP DLDDVARAAE RMVEAEDSAI F HGWAQAGI KGIVDSTPHE ALAVASVSDF PRAVLSAADT LRKAGVTGPY ...String: MDLLKRHLAP IVPDAWSAID EEAKEIFQGH LAGRKLVDFR GPFGWEYAAV NTGELRPIDD TPEDVDMKL RQVQPLAEVR VPFTLDVTEL DSVARGATNP DLDDVARAAE RMVEAEDSAI F HGWAQAGI KGIVDSTPHE ALAVASVSDF PRAVLSAADT LRKAGVTGPY ALVLGPKAYD DL FAATQDG YPVAKQVQRL VVDGPLVRAN ALAGALVMSM RGGDYELTVG QDLSIGYAFH DRS KVELFV AESFTFRVLE PGAAVHLRYA |
-Macromolecule #2: Haliangium ochraceum encapsulated ferritin
| Macromolecule | Name: Haliangium ochraceum encapsulated ferritin / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Haliangium ochraceum (bacteria) Haliangium ochraceum (bacteria) |
| Recombinant expression | Organism:  |
| Sequence | String: MSSEQLHEPA ELLSEETKNM HRALVTLIEE LEAVDWYQQR ADACSEPGLH DVLIHNKNEE VEHAMMTLE WIRRRSPVFD AHMRTYLFTE RPILELEEED TGSSSSVAAS PTSAPSHGSL G IGSLRQEG KED |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: Solutions were prepared with MilliQ water and filtered using a 0.22 um filter. | |||||||||
| Grid | Model: Quantifoil / Material: GOLD / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV Details: blot force -5, wait time 10 seconds and blot time of 3 seconds. | |||||||||
| Details | Sample mono disperse as determined by SEC |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 11520 pixel / Digitization - Dimensions - Height: 8184 pixel / Number grids imaged: 1 / Number real images: 8109 / Average exposure time: 1.0 sec. / Average electron dose: 40.509 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)