[English] 日本語
 Yorodumi
Yorodumi- EMDB-12115: Yeast C complex spliceosome, reconstructed signal-subtracted map ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12115 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Yeast C complex spliceosome, reconstructed signal-subtracted map after focused classification on Prp19 | |||||||||
 Map data Map data | Yeast C complex spliceosome, reconstructed signal-subtracted map after focused classification on Prp19 | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
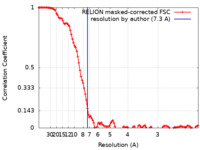
| Method | single particle reconstruction / cryo EM / Resolution: 7.3 Å | |||||||||
 Authors Authors | Wilkinson ME / Fica SM / Galej WP / Nagai K | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2021 Journal: Mol Cell / Year: 2021Title: Structural basis for conformational equilibrium of the catalytic spliceosome. Authors: Max E Wilkinson / Sebastian M Fica / Wojciech P Galej / Kiyoshi Nagai /  Abstract: The ATPase Prp16 governs equilibrium between the branching (B/C) and exon ligation (C/P) conformations of the spliceosome. Here, we present the electron cryomicroscopy reconstruction of the ...The ATPase Prp16 governs equilibrium between the branching (B/C) and exon ligation (C/P) conformations of the spliceosome. Here, we present the electron cryomicroscopy reconstruction of the Saccharomyces cerevisiae C-complex spliceosome at 2.8 Å resolution and identify a novel C-complex intermediate (C) that elucidates the molecular basis for this equilibrium. The exon-ligation factors Prp18 and Slu7 bind to C before ATP hydrolysis by Prp16 can destabilize the branching conformation. Biochemical assays suggest that these pre-bound factors prime the C complex for conversion to C by Prp16. A complete model of the Prp19 complex (NTC) reveals how the branching factors Yju2 and Isy1 are recruited by the NTC before branching. Prp16 remodels Yju2 binding after branching, allowing Yju2 to remain tethered to the NTC in the C complex to promote exon ligation. Our results explain how Prp16 action modulates the dynamic binding of step-specific factors to alternatively stabilize the C or C conformation and establish equilibrium of the catalytic spliceosome. #1:  Journal: To Be Published Journal: To Be PublishedTitle: Structural basis for conformational equilibrium of the catalytic spliceosome Authors: Wilkinson ME / Fica SM / Galej WP / Nagai K | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12115.map.gz emd_12115.map.gz | 94 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12115-v30.xml emd-12115-v30.xml emd-12115.xml emd-12115.xml | 19.4 KB 19.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12115_fsc.xml emd_12115_fsc.xml | 10.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_12115.png emd_12115.png | 90 KB | ||
| Others |  emd_12115_half_map_1.map.gz emd_12115_half_map_1.map.gz emd_12115_half_map_2.map.gz emd_12115_half_map_2.map.gz | 80.6 MB 80.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12115 http://ftp.pdbj.org/pub/emdb/structures/EMD-12115 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12115 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12115 | HTTPS FTP |
-Related structure data
| Related structure data |  7b9vC C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10687 (Title: Yeast C, Ci, C*, and P complex spliceosomes / Data size: 8.9 TB EMPIAR-10687 (Title: Yeast C, Ci, C*, and P complex spliceosomes / Data size: 8.9 TBData #1: Unaligned movies of C-complex spliceosome with 3' splice site AG to AC mutation (Dataset 1) [micrographs - multiframe] Data #2: Unaligned movies of C and C*-complex spliceosomes with 3' splice site AG to AdG mutation (Dataset 2) [micrographs - multiframe] Data #3: Unaligned movies of C and C*-complex spliceosomes with 3' splice site AG to AdG mutation (Dataset 3) [micrographs - multiframe] Data #4: Aligned movies of C-complex spliceosomes with cold-sensitive prp16-302 mutation, purified with Cwc25 (Dataset 4) [micrographs - multiframe] Data #5: Unaligned movies of C-complex spliceosomes with cold-sensitive prp16-302 mutation, purified with Cwc25 and incubated with ATP and Mg (Dataset 5) [micrographs - multiframe] Data #6: Unaligned movies of C, C*, and P-complex spliceosomes with dominant-negative Prp22 mutation K512A, purified with Slu7 (Dataset 6) [micrographs - multiframe] Data #7: Unaligned movies of P-complex spliceosomes with dominant-negative Prp22 mutation K512A, treated with anti-3'exon RNaseH oligo, purified in presence of Mg (Dataset 9) [micrographs - single frame] Data #8: Selected C-complex particles after polishing [picked particles - single frame - processed] Data #9: Selected P-complex particles after polishing [picked particles - single frame - processed] Data #10: Various signal subtractions for C- and P-complex spliceosomes [picked particles - single frame - processed]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_12115.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12115.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Yeast C complex spliceosome, reconstructed signal-subtracted map after focused classification on Prp19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.145 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: Yeast C complex spliceosome, reconstructed signal-subtracted map after...
| File | emd_12115_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Yeast C complex spliceosome, reconstructed signal-subtracted map after focused classification on Prp19, half-map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Yeast C complex spliceosome, reconstructed signal-subtracted map after...
| File | emd_12115_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Yeast C complex spliceosome, reconstructed signal-subtracted map after focused classification on Prp19, half-map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Yeast C complex spliceosome with Prp18/Slu7 bound
| Entire | Name: Yeast C complex spliceosome with Prp18/Slu7 bound |
|---|---|
| Components |
|
-Supramolecule #1: Yeast C complex spliceosome with Prp18/Slu7 bound
| Supramolecule | Name: Yeast C complex spliceosome with Prp18/Slu7 bound / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#40 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 2 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.9 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Support film - Material: CARBON / Support film - topology: CONTINUOUS |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Number grids imaged: 9 / Number real images: 24115 / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)