[English] 日本語
 Yorodumi
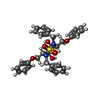
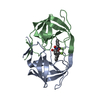
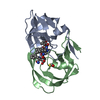
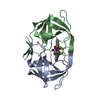
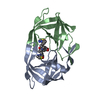
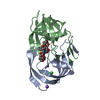
Yorodumi- PDB-1ajv: HIV-1 PROTEASE IN COMPLEX WITH THE CYCLIC SULFAMIDE INHIBITOR AHA006 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ajv | ||||||
|---|---|---|---|---|---|---|---|
| Title | HIV-1 PROTEASE IN COMPLEX WITH THE CYCLIC SULFAMIDE INHIBITOR AHA006 | ||||||
 Components Components | HIV-1 PROTEASE | ||||||
 Keywords Keywords | ASPARTYL PROTEASE / PROTEASE / NON-PEPTIDE INHIBITOR / DRUG DESIGN / HIV-1 | ||||||
| Function / homology |  Function and homology information Function and homology informationHIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding ...HIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / DNA integration / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding / viral penetration into host nucleus / host multivesicular body / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / host cell / viral nucleocapsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / symbiont-mediated suppression of host gene expression / viral translational frameshifting / symbiont entry into host cell / lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / proteolysis / DNA binding / zinc ion binding / membrane Similarity search - Function | ||||||
| Biological species |   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2 Å MOLECULAR REPLACEMENT / Resolution: 2 Å | ||||||
 Authors Authors | Backbro, K. / Unge, T. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 1997 Journal: J.Med.Chem. / Year: 1997Title: Unexpected binding mode of a cyclic sulfamide HIV-1 protease inhibitor. Authors: Backbro, K. / Lowgren, S. / Osterlund, K. / Atepo, J. / Unge, T. / Hulten, J. / Bonham, N.M. / Schaal, W. / Karlen, A. / Hallberg, A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ajv.cif.gz 1ajv.cif.gz | 52.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ajv.ent.gz pdb1ajv.ent.gz | 37.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ajv.json.gz 1ajv.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/aj/1ajv https://data.pdbj.org/pub/pdb/validation_reports/aj/1ajv ftp://data.pdbj.org/pub/pdb/validation_reports/aj/1ajv ftp://data.pdbj.org/pub/pdb/validation_reports/aj/1ajv | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1ajxC  4phvS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 10803.756 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Human immunodeficiency virus 1 / Genus: Lentivirus / Strain: BH10 / Cell line: BL21 / Gene: PROTEASE / Plasmid: PET11C / Species (production host): Escherichia coli / Gene (production host): PROTEASE / Production host: Human immunodeficiency virus 1 / Genus: Lentivirus / Strain: BH10 / Cell line: BL21 / Gene: PROTEASE / Plasmid: PET11C / Species (production host): Escherichia coli / Gene (production host): PROTEASE / Production host:  #2: Chemical | ChemComp-NMB / | #3: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.8 Å3/Da / Density % sol: 54.03 % | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion / pH: 5.5 Details: CRYSTALLIZATION WAS PERFORMED BY VAPOR DIFFUSION. PROTEASE (2 MG/ML) AND INHIBITOR (40 MM IN DMSO) WAS MIXED IN A RATIO OF 1:1 AND SUBJECTED TO COCRYSTALLIZATION. DROPS CONSISTING OF 5 ...Details: CRYSTALLIZATION WAS PERFORMED BY VAPOR DIFFUSION. PROTEASE (2 MG/ML) AND INHIBITOR (40 MM IN DMSO) WAS MIXED IN A RATIO OF 1:1 AND SUBJECTED TO COCRYSTALLIZATION. DROPS CONSISTING OF 5 MICROLITERS OF THE PROTEASE-INHIBITOR MIXTURE PLUS 5 MICROLITERS OF THE CRYSTALLIZATION BUFFER (50 MM MES PH 5.5, 0.4 M NACL AND 0.02% (W/V) NAN3) WERE EQUILIBRATED AGAINST THE SAME BUFFER AT 4 DEGREES C., vapor diffusion, temperature 277K | ||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 4 ℃ / Method: vapor diffusionDetails: drop solution was mixed with an equal volume of reservoir solution | ||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 278 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: LURE SYNCHROTRON / Site: LURE  / Beamline: DW32 / Wavelength: 0.9 / Beamline: DW32 / Wavelength: 0.9 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: May 1, 1995 |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9 Å / Relative weight: 1 |
| Reflection | Resolution: 2→25 Å / Num. obs: 14846 / % possible obs: 91.7 % / Observed criterion σ(I): 2 / Rmerge(I) obs: 0.041 |
| Reflection shell | Resolution: 2→2.07 Å / Rmerge(I) obs: 0.088 / % possible all: 80 |
| Reflection | *PLUS Num. measured all: 73708 |
| Reflection shell | *PLUS % possible obs: 80 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 4PHV Resolution: 2→8 Å / Cross valid method: THROUGHOUT / σ(F): 2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 17 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2→2.07 Å / Total num. of bins used: 10
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.1F / Classification: refinement X-PLOR / Version: 3.1F / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Rfactor obs: 0.088 |
 Movie
Movie Controller
Controller

















 PDBj
PDBj