[English] 日本語
 Yorodumi
Yorodumi- PDB-1ahz: STRUCTURE OF THE OCTAMERIC FLAVOENZYME VANILLYL-ALCOHOL OXIDASE I... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ahz | ||||||
|---|---|---|---|---|---|---|---|
| Title | STRUCTURE OF THE OCTAMERIC FLAVOENZYME VANILLYL-ALCOHOL OXIDASE IN COMPLEX WITH 4-(1-HEPTENYL)PHENOL | ||||||
 Components Components | VANILLYL-ALCOHOL OXIDASE | ||||||
 Keywords Keywords | FLAVOENZYME / OXIDASE / CATALYSIS | ||||||
| Function / homology |  Function and homology information Function and homology informationvanillyl-alcohol oxidase / vanillyl-alcohol oxidase activity / methanol metabolic process / lactate catabolic process / D-lactate dehydrogenase (cytochrome) activity / D-lactate dehydrogenase (NAD+) activity / FAD binding / peroxisome / mitochondrion Similarity search - Function | ||||||
| Biological species |  Penicillium simplicissimum (fungus) Penicillium simplicissimum (fungus) | ||||||
| Method |  X-RAY DIFFRACTION / DENSITY AVERAGING, LEAST SQUARES REFINEMENT / Resolution: 3.3 Å X-RAY DIFFRACTION / DENSITY AVERAGING, LEAST SQUARES REFINEMENT / Resolution: 3.3 Å | ||||||
 Authors Authors | Mattevi, A. | ||||||
 Citation Citation |  Journal: Structure / Year: 1997 Journal: Structure / Year: 1997Title: Crystal structures and inhibitor binding in the octameric flavoenzyme vanillyl-alcohol oxidase: the shape of the active-site cavity controls substrate specificity. Authors: Mattevi, A. / Fraaije, M.W. / Mozzarelli, A. / Olivi, L. / Coda, A. / van Berkel, W.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ahz.cif.gz 1ahz.cif.gz | 226.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ahz.ent.gz pdb1ahz.ent.gz | 182.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ahz.json.gz 1ahz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ah/1ahz https://data.pdbj.org/pub/pdb/validation_reports/ah/1ahz ftp://data.pdbj.org/pub/pdb/validation_reports/ah/1ahz ftp://data.pdbj.org/pub/pdb/validation_reports/ah/1ahz | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 |
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (0.30602, -0.95098, 0.04465), Vector: |
- Components
Components
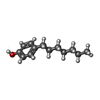
| #1: Protein | Mass: 63107.121 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Details: FUNGUS / Source: (natural)  Penicillium simplicissimum (fungus) / Cellular location: INTRACELLULAR / Organelle: PEROXISOMES / References: UniProt: P56216, alcohol oxidase Penicillium simplicissimum (fungus) / Cellular location: INTRACELLULAR / Organelle: PEROXISOMES / References: UniProt: P56216, alcohol oxidase#2: Chemical | #3: Chemical | #4: Chemical | ChemComp-EPT / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.3 Å3/Da / Density % sol: 40 % / Description: BINDING STUDIES | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 4.6 / Details: FROM 6% PEG4000, 100 MM ACETATE BUFFER PH 4.6 | |||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: vapor diffusion, hanging drop | |||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 300 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RUH2R / Wavelength: 1.5418 |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Nov 1, 1996 / Details: COLLIMATOR |
| Radiation | Monochromator: GRAPHITE(002) / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 3.3→30 Å / Num. obs: 17633 / % possible obs: 90.7 % / Observed criterion σ(I): 0 / Redundancy: 2 % / Biso Wilson estimate: 24 Å2 / Rsym value: 0.111 / Net I/σ(I): 8 |
| Reflection shell | Resolution: 3.3→3.4 Å / Redundancy: 2 % / Rmerge(I) obs: 0.339 / Mean I/σ(I) obs: 3 / Rsym value: 0.339 / % possible all: 86.1 |
| Reflection | *PLUS Num. measured all: 41329 / Rmerge(I) obs: 0.111 |
| Reflection shell | *PLUS % possible obs: 86.1 % |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure: DENSITY AVERAGING, LEAST SQUARES REFINEMENT Resolution: 3.3→30 Å / Isotropic thermal model: TNT BCORREL / σ(F): 0 / Stereochemistry target values: TNT PROTGEO
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: BABINET | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.3→30 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: TNT / Version: 5-E / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Rfactor Rfree: 0.268 / Lowest resolution: 100 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller
















 PDBj
PDBj