[English] 日本語
 Yorodumi
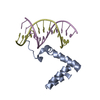
Yorodumi- PDB-1afo: DIMERIC TRANSMEMBRANE DOMAIN OF HUMAN GLYCOPHORIN A, NMR, 20 STRU... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1afo | ||||||
|---|---|---|---|---|---|---|---|
| Title | DIMERIC TRANSMEMBRANE DOMAIN OF HUMAN GLYCOPHORIN A, NMR, 20 STRUCTURES | ||||||
 Components Components | GLYCOPHORIN A | ||||||
 Keywords Keywords | INTEGRAL MEMBRANE PROTEIN / HUMAN GLYCOPHORIN A / TRANSMEMBRANE HELIX INTERACTIONS / MEMBRANE PROTEIN FOLDING | ||||||
| Function / homology |  Function and homology information Function and homology informationankyrin-1 complex / Cell surface interactions at the vascular wall / virus receptor activity / nucleoplasm / identical protein binding / membrane / plasma membrane / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | SOLUTION NMR / DISTANCE GEOMETRY SIMULATED ANNEALING HYBRID | ||||||
 Authors Authors | Mackenzie, K.R. / Prestegard, J.H. / Engelman, D.M. | ||||||
 Citation Citation |  Journal: Science / Year: 1997 Journal: Science / Year: 1997Title: A transmembrane helix dimer: structure and implications. Authors: MacKenzie, K.R. / Prestegard, J.H. / Engelman, D.M. #1:  Journal: Thesis, Yale University / Year: 1996 Journal: Thesis, Yale University / Year: 1996Title: Structure Determination of the Dimeric Membrane Spanning Domain of Glycophorin a in Detergent Micelles by Triple Resonance Nuclear Magnetic Resonance Spectroscopy Authors: Mackenzie, K.R. #2:  Journal: J.Biomol.NMR / Year: 1996 Journal: J.Biomol.NMR / Year: 1996Title: Leucine Side-Chain Rotamers in a Glycophorin a Transmembrane Peptide as Revealed by Three-Bond Carbon-Carbon Couplings and 13C Chemical Shifts Authors: Mackenzie, K.R. / Prestegard, J.H. / Engelman, D.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1afo.cif.gz 1afo.cif.gz | 575.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1afo.ent.gz pdb1afo.ent.gz | 493.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1afo.json.gz 1afo.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  1afo_validation.pdf.gz 1afo_validation.pdf.gz | 362.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  1afo_full_validation.pdf.gz 1afo_full_validation.pdf.gz | 587 KB | Display | |
| Data in XML |  1afo_validation.xml.gz 1afo_validation.xml.gz | 37.3 KB | Display | |
| Data in CIF |  1afo_validation.cif.gz 1afo_validation.cif.gz | 59 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/af/1afo https://data.pdbj.org/pub/pdb/validation_reports/af/1afo ftp://data.pdbj.org/pub/pdb/validation_reports/af/1afo ftp://data.pdbj.org/pub/pdb/validation_reports/af/1afo | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 4454.414 Da / Num. of mol.: 2 / Fragment: TRANSMEMBRANE PEPTIDE Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Strain: MG-T7 / Cell: ERYTHROCYTE / Cellular location: PLASMA MEMBRANE / Organ: PLASMA / Plasmid: PET3A / Production host: Homo sapiens (human) / Strain: MG-T7 / Cell: ERYTHROCYTE / Cellular location: PLASMA MEMBRANE / Organ: PLASMA / Plasmid: PET3A / Production host:  |
|---|
-Experimental details
-Experiment
| Experiment | Method: SOLUTION NMR | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Sample conditions | pH: 6 / Temperature: 313 K |
|---|---|
| Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| NMR spectrometer |
|
|---|
- Processing
Processing
| Software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR software |
| ||||||||||||
| Refinement | Method: DISTANCE GEOMETRY SIMULATED ANNEALING HYBRID / Software ordinal: 1 | ||||||||||||
| NMR ensemble | Conformer selection criteria: LEAST RESTRAINT VIOLATIONS / Conformers calculated total number: 38 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller









 PDBj
PDBj HNCA
HNCA