+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 8jjl | ||||||
|---|---|---|---|---|---|---|---|
| タイトル | cryo-EM structure of the beta2-AR-mBRIL/1b3 Fab/Glue complex with a full agonist | ||||||
 要素 要素 | Beta-2 adrenergic receptor,Soluble cytochrome b562 | ||||||
 キーワード キーワード | MEMBRANE PROTEIN / complex | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報beta2-adrenergic receptor activity / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of mini excitatory postsynaptic potential / positive regulation of cAMP-dependent protein kinase activity / positive regulation of AMPA receptor activity / norepinephrine binding / positive regulation of autophagosome maturation / heat generation / Adrenoceptors / activation of transmembrane receptor protein tyrosine kinase activity ...beta2-adrenergic receptor activity / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of mini excitatory postsynaptic potential / positive regulation of cAMP-dependent protein kinase activity / positive regulation of AMPA receptor activity / norepinephrine binding / positive regulation of autophagosome maturation / heat generation / Adrenoceptors / activation of transmembrane receptor protein tyrosine kinase activity / negative regulation of smooth muscle contraction / positive regulation of lipophagy / negative regulation of multicellular organism growth / negative regulation of G protein-coupled receptor signaling pathway / response to psychosocial stress / endosome to lysosome transport / adrenergic receptor signaling pathway / diet induced thermogenesis / positive regulation of protein kinase A signaling / neuronal dense core vesicle / adenylate cyclase binding / smooth muscle contraction / bone resorption / potassium channel regulator activity / positive regulation of bone mineralization / brown fat cell differentiation / adenylate cyclase-activating adrenergic receptor signaling pathway / regulation of sodium ion transport / response to cold / receptor-mediated endocytosis / electron transport chain / clathrin-coated endocytic vesicle membrane / positive regulation of protein serine/threonine kinase activity / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / cellular response to amyloid-beta / Cargo recognition for clathrin-mediated endocytosis / positive regulation of cold-induced thermogenesis / Clathrin-mediated endocytosis / amyloid-beta binding / G alpha (s) signalling events / transcription by RNA polymerase II / positive regulation of MAPK cascade / periplasmic space / lysosome / electron transfer activity / early endosome / receptor complex / cell surface receptor signaling pathway / endosome membrane / Ub-specific processing proteases / endosome / iron ion binding / apical plasma membrane / heme binding / protein-containing complex binding / Golgi apparatus / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / identical protein binding / membrane / nucleus / plasma membrane 類似検索 - 分子機能 | ||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | ||||||
| 手法 | 電子顕微鏡法 / 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.2 Å | ||||||
 データ登録者 データ登録者 | He, B.B. / Zhong, Y.X. / Guo, Q. / Tao, Y.Y. | ||||||
| 資金援助 |  中国, 1件 中国, 1件
| ||||||
 引用 引用 |  ジャーナル: Nat Chem Biol / 年: 2024 ジャーナル: Nat Chem Biol / 年: 2024タイトル: A method for structure determination of GPCRs in various states. 著者: Qiong Guo / Binbin He / Yixuan Zhong / Haizhan Jiao / Yinhang Ren / Qinggong Wang / Qiangqiang Ge / Yongxiang Gao / Xiangyu Liu / Yang Du / Hongli Hu / Yuyong Tao /  要旨: G-protein-coupled receptors (GPCRs) are a class of integral membrane proteins that detect environmental cues and trigger cellular responses. Deciphering the functional states of GPCRs induced by ...G-protein-coupled receptors (GPCRs) are a class of integral membrane proteins that detect environmental cues and trigger cellular responses. Deciphering the functional states of GPCRs induced by various ligands has been one of the primary goals in the field. Here we developed an effective universal method for GPCR cryo-electron microscopy structure determination without the need to prepare GPCR-signaling protein complexes. Using this method, we successfully solved the structures of the β-adrenergic receptor (βAR) bound to antagonistic and agonistic ligands and the adhesion GPCR ADGRL3 in the apo state. For βAR, an intermediate state stabilized by the partial agonist was captured. For ADGRL3, the structure revealed that inactive ADGRL3 adopts a compact fold and that large unusual conformational changes on both the extracellular and intracellular sides are required for activation of adhesion GPCRs. We anticipate that this method will open a new avenue for understanding GPCR structure‒function relationships and drug development. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  8jjl.cif.gz 8jjl.cif.gz | 86.8 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb8jjl.ent.gz pdb8jjl.ent.gz | 63 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  8jjl.json.gz 8jjl.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  8jjl_validation.pdf.gz 8jjl_validation.pdf.gz | 466.1 KB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  8jjl_full_validation.pdf.gz 8jjl_full_validation.pdf.gz | 470.9 KB | 表示 | |
| XML形式データ |  8jjl_validation.xml.gz 8jjl_validation.xml.gz | 9.4 KB | 表示 | |
| CIF形式データ |  8jjl_validation.cif.gz 8jjl_validation.cif.gz | 13.6 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/jj/8jjl https://data.pdbj.org/pub/pdb/validation_reports/jj/8jjl ftp://data.pdbj.org/pub/pdb/validation_reports/jj/8jjl ftp://data.pdbj.org/pub/pdb/validation_reports/jj/8jjl | HTTPS FTP |
-関連構造データ
| 関連構造データ |  36360MC  8j7eC  8jj8C  8jjoC  8jmtC M: このデータのモデリングに利用したマップデータ C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
|
|---|---|
| 1 |
|
- 要素
要素
| #1: タンパク質 | 分子量: 51594.102 Da / 分子数: 1 / 由来タイプ: 組換発現 由来: (組換発現)  Homo sapiens (ヒト), (組換発現) Homo sapiens (ヒト), (組換発現)  遺伝子: ADRB2, ADRB2R, B2AR, cybC / 発現宿主:  Homo sapiens (ヒト) / 参照: UniProt: P07550, UniProt: P0ABE7 Homo sapiens (ヒト) / 参照: UniProt: P07550, UniProt: P0ABE7 |
|---|---|
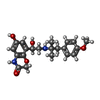
| #2: 化合物 | ChemComp-DZQ / |
| 研究の焦点であるリガンドがあるか | Y |
| Has protein modification | Y |
-実験情報
-実験
| 実験 | 手法: 電子顕微鏡法 |
|---|---|
| EM実験 | 試料の集合状態: CELL / 3次元再構成法: 単粒子再構成法 |
- 試料調製
試料調製
| 構成要素 | 名称: chimeric beta-2AR adrenergic receptor / タイプ: COMPLEX / Entity ID: #1 / 由来: RECOMBINANT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 由来(天然) |
| ||||||||||||
| 由来(組換発現) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) | ||||||||||||
| 緩衝液 | pH: 7.5 | ||||||||||||
| 試料 | 包埋: NO / シャドウイング: NO / 染色: NO / 凍結: YES | ||||||||||||
| 試料支持 | グリッドの材料: NICKEL/TITANIUM | ||||||||||||
| 急速凍結 | 凍結剤: NITROGEN |
- 電子顕微鏡撮影
電子顕微鏡撮影
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
|---|---|
| 顕微鏡 | モデル: FEI TITAN KRIOS |
| 電子銃 | 電子線源:  FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM |
| 電子レンズ | モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 5000 nm / 最小 デフォーカス(公称値): 1200 nm |
| 撮影 | 電子線照射量: 52 e/Å2 フィルム・検出器のモデル: GATAN K3 BIOQUANTUM (6k x 4k) |
- 解析
解析
| CTF補正 | タイプ: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
| 3次元再構成 | 解像度: 3.2 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 粒子像の数: 785234 / 対称性のタイプ: POINT |
 ムービー
ムービー コントローラー
コントローラー






 PDBj
PDBj