[English] 日本語
 Yorodumi
Yorodumi- EMDB-8921: Cryo-EM structure of mouse TRPV3-Y564A in complex with 2-Aminoeth... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8921 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of mouse TRPV3-Y564A in complex with 2-Aminoethoxydiphenyl borate (2-APB) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TRPV3 / TRP channels / Calcium channels / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of hair cycle / TRP channels / response to temperature stimulus / positive regulation of calcium ion import / sodium channel activity / monoatomic ion channel activity / monoatomic cation channel activity / calcium channel activity / lysosome / receptor complex ...negative regulation of hair cycle / TRP channels / response to temperature stimulus / positive regulation of calcium ion import / sodium channel activity / monoatomic ion channel activity / monoatomic cation channel activity / calcium channel activity / lysosome / receptor complex / metal ion binding / identical protein binding / membrane / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.24 Å | |||||||||
 Authors Authors | Singh AK / McGoldrick LL | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2018 Journal: Nat Struct Mol Biol / Year: 2018Title: Structure and gating mechanism of the transient receptor potential channel TRPV3. Authors: Appu K Singh / Luke L McGoldrick / Alexander I Sobolevsky /  Abstract: Transient receptor potential vanilloid subfamily member 3 (TRPV3) channel plays a crucial role in skin physiology and pathophysiology. Mutations in TRPV3 are associated with various skin diseases, ...Transient receptor potential vanilloid subfamily member 3 (TRPV3) channel plays a crucial role in skin physiology and pathophysiology. Mutations in TRPV3 are associated with various skin diseases, including Olmsted syndrome, atopic dermatitis, and rosacea. Here we present the cryo-electron microscopy structures of full-length mouse TRPV3 in the closed apo and agonist-bound open states. The agonist binds three allosteric sites distal to the pore. Channel opening is accompanied by conformational changes in both the outer pore and the intracellular gate. The gate is formed by the pore-lining S6 helices that undergo local α-to-π helical transitions, elongate, rotate, and splay apart in the open state. In the closed state, the shorter S6 segments are entirely α-helical, expose their nonpolar surfaces to the pore, and hydrophobically seal the ion permeation pathway. These findings further illuminate TRP channel activation and can aid in the design of drugs for the treatment of inflammatory skin conditions, itch, and pain. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8921.map.gz emd_8921.map.gz | 7.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8921-v30.xml emd-8921-v30.xml emd-8921.xml emd-8921.xml | 16.4 KB 16.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8921.png emd_8921.png | 243.6 KB | ||
| Filedesc metadata |  emd-8921.cif.gz emd-8921.cif.gz | 6.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8921 http://ftp.pdbj.org/pub/emdb/structures/EMD-8921 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8921 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8921 | HTTPS FTP |
-Validation report
| Summary document |  emd_8921_validation.pdf.gz emd_8921_validation.pdf.gz | 432.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_8921_full_validation.pdf.gz emd_8921_full_validation.pdf.gz | 432.5 KB | Display | |
| Data in XML |  emd_8921_validation.xml.gz emd_8921_validation.xml.gz | 6 KB | Display | |
| Data in CIF |  emd_8921_validation.cif.gz emd_8921_validation.cif.gz | 6.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8921 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8921 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8921 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8921 | HTTPS FTP |
-Related structure data
| Related structure data |  6dvzMC  8919C  8920C  8925C  6dvwC  6dvyC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8921.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8921.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
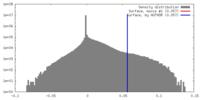
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : TRPV3-Y564A
| Entire | Name: TRPV3-Y564A |
|---|---|
| Components |
|
-Supramolecule #1: TRPV3-Y564A
| Supramolecule | Name: TRPV3-Y564A / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Transient receptor potential cation channel subfamily V member 3
| Macromolecule | Name: Transient receptor potential cation channel subfamily V member 3 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 90.615422 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGAHSKEMAP LMGKRTTAPG GNPVVLTEKR PADLTPTKKS AHFFLEIEGF EPNPTVTKTS PPIFSKPMDS NIRQCLSGNC DDMDSPQSP QDDVTETPSN PNSPSANLAK EEQRQKKKRL KKRIFAAVSE GCVEELRELL QDLQDLCRRR RGLDVPDFLM H KLTASDTG ...String: MGAHSKEMAP LMGKRTTAPG GNPVVLTEKR PADLTPTKKS AHFFLEIEGF EPNPTVTKTS PPIFSKPMDS NIRQCLSGNC DDMDSPQSP QDDVTETPSN PNSPSANLAK EEQRQKKKRL KKRIFAAVSE GCVEELRELL QDLQDLCRRR RGLDVPDFLM H KLTASDTG KTCLMKALLN INPNTKEIVR ILLAFAEEND ILDRFINAEY TEEAYEGQTA LNIAIERRQG DITAVLIAAG AD VNAHAKG VFFNPKYQHE GFYFGETPLA LAACTNQPEI VQLLMENEQT DITSQDSRGN NILHALVTVA EDFKTQNDFV KRM YDMILL RSGNWELETM RNNDGLTPLQ LAAKMGKAEI LKYILSREIK EKPLRSLSRK FTDWAYGPVS SSLYDLTNVD TTTD NSVLE IIVYNTNIDN RHEMLTLEPL HTLLHTKWKK FAKYMFFLSF CFYFFYNITL TLVSYYRPRE DEDLPHPLAL THKMS WLQL LGRMFVLIWA TCISVKEGIA IFLLRPSDLQ SILSDAWFHF VFFVQAVLVI LSVFLYLFAY KEYLACLVLA MALGWA NML AYTRGFQSMG MYSVMIQKVI LHDVLKFLFV YILFLLGFGV ALASLIEKCS KDKKDCSSYG SFSDAVLELF KLTIGLG DL NIQQNSTYPI LFLFLLITYV ILTFVLLLNM LIALMGETVE NVSKESERIW RLQRARTILE FEKMLPEWLR SRFRMGEL C KVADEDFRLC LRINEVKWTE WKTHVSFLNE DPGPIRRTAD LNKIQDSSRS NSKTTLYAFD ELDEFPETSV UniProtKB: Transient receptor potential cation channel subfamily V member 3 |
-Macromolecule #2: 2-aminoethyl diphenylborinate
| Macromolecule | Name: 2-aminoethyl diphenylborinate / type: ligand / ID: 2 / Number of copies: 12 / Formula: FZ4 |
|---|---|
| Molecular weight | Theoretical: 225.094 Da |
| Chemical component information |  ChemComp-FZ4: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.0 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Material: GOLD / Support film - Material: CARBON / Support film - topology: HOLEY | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)