[English] 日本語
 Yorodumi
Yorodumi- EMDB-8854: Structure of the major capsid protein and the capsid stabilizing ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8854 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
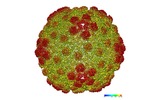
| Title | Structure of the major capsid protein and the capsid stabilizing protein of the marine siphovirus TW1 | |||||||||
 Map data Map data | icosahedral reconstruction of marine Siphophage TW1 head | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Major Capsid Protein / Capsid Stabilizing Protein / HK 97 fold / Decoration Protein / VIRUS | |||||||||
| Function / homology | Protein of unknown function DUF6260 / Family of unknown function (DUF6260) / viral capsid / Putative coat protein / Uncharacterized protein Function and homology information Function and homology information | |||||||||
| Biological species |  Pseudoalteromonas phage TW1 (virus) Pseudoalteromonas phage TW1 (virus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Wang Z / Rossmann MG | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2018 Journal: Structure / Year: 2018Title: Structure of the Marine Siphovirus TW1: Evolution of Capsid-Stabilizing Proteins and Tail Spikes. Authors: Zhiqing Wang / Stephen C Hardies / Andrei Fokine / Thomas Klose / Wen Jiang / Byung Cheol Cho / Michael G Rossmann /   Abstract: Marine bacteriophage TW1 belongs to the Siphoviridae family and infects Pseudoalteromonas phenolica. Mass spectrometry analysis has identified 16 different proteins in the TW1 virion. Functions of ...Marine bacteriophage TW1 belongs to the Siphoviridae family and infects Pseudoalteromonas phenolica. Mass spectrometry analysis has identified 16 different proteins in the TW1 virion. Functions of most of these proteins have been predicted by bioinformatic methods. A 3.6 Å resolution cryoelectron microscopy map of the icosahedrally averaged TW1 head showed the atomic structures of the major capsid protein, gp57, and the capsid-stabilizing protein, gp56. The gp57 structure is similar to that of the phage HK97 capsid protein. The gp56 protein has two domains, each having folds similar to that of the N-terminal part of phage λ gpD, indicating a common ancestry. The first gp56 domain clamps adjacent capsomers together, whereas the second domain is required for trimerization. A 6-fold-averaged reconstruction of the distal part of the tail showed that TW1 has six tail spikes, which are unusual for siphophages but are similar to the podophages P22 and Sf6, suggesting a common evolutionary origin of these spikes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8854.map.gz emd_8854.map.gz | 2.9 GB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8854-v30.xml emd-8854-v30.xml emd-8854.xml emd-8854.xml | 13.5 KB 13.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8854.png emd_8854.png | 297.7 KB | ||
| Filedesc metadata |  emd-8854.cif.gz emd-8854.cif.gz | 5.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8854 http://ftp.pdbj.org/pub/emdb/structures/EMD-8854 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8854 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8854 | HTTPS FTP |
-Related structure data
| Related structure data |  5wk1MC  7070C  8867C  8868C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_8854.map.gz / Format: CCP4 / Size: 11.1 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8854.map.gz / Format: CCP4 / Size: 11.1 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | icosahedral reconstruction of marine Siphophage TW1 head | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.667 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
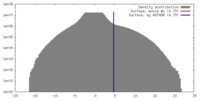
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Pseudoalteromonas phage TW1
| Entire | Name:  Pseudoalteromonas phage TW1 (virus) Pseudoalteromonas phage TW1 (virus) |
|---|---|
| Components |
|
-Supramolecule #1: Pseudoalteromonas phage TW1
| Supramolecule | Name: Pseudoalteromonas phage TW1 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 1366055 / Sci species name: Pseudoalteromonas phage TW1 / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|
-Macromolecule #1: Capsid Stabilizing Protein
| Macromolecule | Name: Capsid Stabilizing Protein / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudoalteromonas phage TW1 (virus) Pseudoalteromonas phage TW1 (virus) |
| Molecular weight | Theoretical: 15.017777 KDa |
| Sequence | String: MANSKNSIFV GGAGRVKQTI EGLAQSAFKP GQLLARAAGD AIDVTAKAST TYGNEFLICD DQPQTLGGGT DVAVTAGDTV QAISVLPGQ YVLLSFAATQ NVTTKGAAVA SNGDGNFKLG NPATEQTFAV TEEIINVTTA GTLVLCRAI UniProtKB: Uncharacterized protein |
-Macromolecule #2: Major Capsid Protein
| Macromolecule | Name: Major Capsid Protein / type: protein_or_peptide / ID: 2 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudoalteromonas phage TW1 (virus) Pseudoalteromonas phage TW1 (virus) |
| Molecular weight | Theoretical: 39.047965 KDa |
| Sequence | String: MSIHFDFKNK QGKELLALNR QWNELSGYRA HAFNESVNML NNVGETFGVN GANAMQTNMQ RVDEMYRLVD STGTGEDRDW GNQTLLGRL LSQAQTVSIG KKVIESRRYS EAGRINRSMS GQTDIDMDKT KSSYQKMVIP VFDGAYGRDF RDYEAMRSEM L PALAEDSE ...String: MSIHFDFKNK QGKELLALNR QWNELSGYRA HAFNESVNML NNVGETFGVN GANAMQTNMQ RVDEMYRLVD STGTGEDRDW GNQTLLGRL LSQAQTVSIG KKVIESRRYS EAGRINRSMS GQTDIDMDKT KSSYQKMVIP VFDGAYGRDF RDYEAMRSEM L PALAEDSE EIEFTLLDDV NDYLWNGDAK LKVDTAVWGG LKADASVAAY SLGADLTTAT EAQVVAELLA LLDVLRITNK KS GPFELYI SPQIMSNWQK LAGANTNGFM NIMAAVRALI PEFSVVEADS ALQGNQVLCS VVGTRGLHAK IGMMMSSYQV PRV MHNDPY QFVKWFAAGF QSNNSFSGLK STVYGS UniProtKB: Putative coat protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
Details: 50 mM Tris, pH 7.5, 100 mM NaCl, 8 mM MgSO4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Ted Pella Inc, Lacey Carbon / Mesh: 400 / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 75 % / Chamber temperature: 298 K / Instrument: GATAN CRYOPLUNGE 3 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 80.0 K / Max: 80.0 K |
| Image recording | Film or detector model: DIRECT ELECTRON DE-16 (4k x 4k) / Detector mode: COUNTING / Average electron dose: 20.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)