[English] 日本語
 Yorodumi
Yorodumi- EMDB-8184: CryoEM structure of the native empty particle of a human rhinovirus C -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8184 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of the native empty particle of a human rhinovirus C | |||||||||
 Map data Map data | CryoEM structure of the native empty particle of a human rhinovirus C | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | virus / jelly roll | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / ribonucleoside triphosphate phosphatase activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / channel activity ...symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / ribonucleoside triphosphate phosphatase activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / DNA replication / RNA helicase activity / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |  Rhinovirus C / Rhinovirus C /  Rhinovirus C15a Rhinovirus C15a | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.16 Å | |||||||||
 Authors Authors | Liu Y / Hill MG | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2016 Journal: Proc Natl Acad Sci U S A / Year: 2016Title: Atomic structure of a rhinovirus C, a virus species linked to severe childhood asthma. Authors: Yue Liu / Marchel G Hill / Thomas Klose / Zhenguo Chen / Kelly Watters / Yury A Bochkov / Wen Jiang / Ann C Palmenberg / Michael G Rossmann /  Abstract: Isolates of rhinovirus C (RV-C), a recently identified Enterovirus (EV) species, are the causative agents of severe respiratory infections among children and are linked to childhood asthma ...Isolates of rhinovirus C (RV-C), a recently identified Enterovirus (EV) species, are the causative agents of severe respiratory infections among children and are linked to childhood asthma exacerbations. The RV-C have been refractory to structure determination because they are difficult to propagate in vitro. Here, we report the cryo-EM atomic structures of the full virion and native empty particle (NEP) of RV-C15a. The virus has 60 "fingers" on the virus outer surface that probably function as dominant immunogens. Because the NEPs also display these fingers, they may have utility as vaccine candidates. A sequence-conserved surface depression adjacent to each finger forms a likely binding site for the sialic acid on its receptor. The RV-C, unlike other EVs, are resistant to capsid-binding antiviral compounds because the hydrophobic pocket in VP1 is filled with multiple bulky residues. These results define potential molecular determinants for designing antiviral therapeutics and vaccines. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8184.map.gz emd_8184.map.gz | 395.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8184-v30.xml emd-8184-v30.xml emd-8184.xml emd-8184.xml | 19.1 KB 19.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_8184_fsc.xml emd_8184_fsc.xml | 19.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_8184.png emd_8184.png | 260.2 KB | ||
| Filedesc metadata |  emd-8184.cif.gz emd-8184.cif.gz | 7.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8184 http://ftp.pdbj.org/pub/emdb/structures/EMD-8184 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8184 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8184 | HTTPS FTP |
-Validation report
| Summary document |  emd_8184_validation.pdf.gz emd_8184_validation.pdf.gz | 614.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_8184_full_validation.pdf.gz emd_8184_full_validation.pdf.gz | 613.9 KB | Display | |
| Data in XML |  emd_8184_validation.xml.gz emd_8184_validation.xml.gz | 15.5 KB | Display | |
| Data in CIF |  emd_8184_validation.cif.gz emd_8184_validation.cif.gz | 20.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8184 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8184 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8184 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8184 | HTTPS FTP |
-Related structure data
| Related structure data |  5jzgMC  8189C  5k0uC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8184.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8184.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM structure of the native empty particle of a human rhinovirus C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
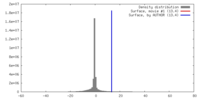
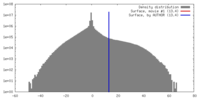
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Rhinovirus C15a
| Entire | Name:  Rhinovirus C15a Rhinovirus C15a |
|---|---|
| Components |
|
-Supramolecule #1: Rhinovirus C15a
| Supramolecule | Name: Rhinovirus C15a / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all Details: The cDNA of RV-C15 isolate was used to produce RNA transcripts in vitro, which were then used for transfection in HeLa WisL cells. The resultant recombinant RV-C15 virus was adapted in HeLa ...Details: The cDNA of RV-C15 isolate was used to produce RNA transcripts in vitro, which were then used for transfection in HeLa WisL cells. The resultant recombinant RV-C15 virus was adapted in HeLa cells expressing the RV-C receptor CDHR3 via multiple passage. The derivative was the RV-C15a virus. NCBI-ID: 463676 / Sci species name: Rhinovirus C15a / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: Yes |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 5.8 MDa |
| Virus shell | Shell ID: 1 / Name: Capsid (p3) / Diameter: 300.0 Å / T number (triangulation number): 3 |
-Macromolecule #1: Capsid protein VP1
| Macromolecule | Name: Capsid protein VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Rhinovirus C Rhinovirus C |
| Molecular weight | Theoretical: 31.802623 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: NNDDPVENFV ESTLKEVLVV PDTKPSGPQH TTKPSILGAM EIGASSNATP ESTIETRYVY NTNTNAEADV EMFLGRSALW GKVTLTRQY AKWEINFQEQ AHIRKKFEFF TYLRFDMEVT IVTNNKGLMQ IMFVPPGIDH PETHDDRKWD SASNPSVFFQ P KSGFPRFT ...String: NNDDPVENFV ESTLKEVLVV PDTKPSGPQH TTKPSILGAM EIGASSNATP ESTIETRYVY NTNTNAEADV EMFLGRSALW GKVTLTRQY AKWEINFQEQ AHIRKKFEFF TYLRFDMEVT IVTNNKGLMQ IMFVPPGIDH PETHDDRKWD SASNPSVFFQ P KSGFPRFT IPFTGLASAY YMFYDGYDKP KGSDNNEYGI APTNDMGLLC FRTLDNSGGN DVKIYVKPKH ITAWVPRPPR AT QYTHKYS TNYHYKPNSS GPDEHVLKDR HFIKTRPLIS SA UniProtKB: Genome polyprotein |
-Macromolecule #2: Capsid protein VP3
| Macromolecule | Name: Capsid protein VP3 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Rhinovirus C Rhinovirus C |
| Molecular weight | Theoretical: 25.965037 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GLPTRLPSGS QQFMTTEDEQ SPNILPGFHP SKKIHIPGMI TNVMHMARVD SFIPINNIQG EVGKVSMYYI TVTKKTVTER ILVLPLEMS NTLFATTLLG EVLNYYANWS GSITITFMCV CDAFSTGKFL VAYTPPGGKL PEDRKQAMLG VHIIWDLGLQ S SCTIVVPW ...String: GLPTRLPSGS QQFMTTEDEQ SPNILPGFHP SKKIHIPGMI TNVMHMARVD SFIPINNIQG EVGKVSMYYI TVTKKTVTER ILVLPLEMS NTLFATTLLG EVLNYYANWS GSITITFMCV CDAFSTGKFL VAYTPPGGKL PEDRKQAMLG VHIIWDLGLQ S SCTIVVPW ISSGFYRRTK ADSFTHGGYV SLWYQTAFVP PVSGGTGSIL ATCSACPDMS VRMLRDSPMM EQKNELQ UniProtKB: Genome polyprotein |
-Macromolecule #3: Capsid protein VP0
| Macromolecule | Name: Capsid protein VP0 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Rhinovirus C Rhinovirus C |
| Molecular weight | Theoretical: 36.247344 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GAQVSRQNNG THENGVTASN GSVIKYFNIN YYKDSASSGL SRQDFSQDPS KFTQPLVDTL TNPALMSPSV EACGYSDRLK QITIGNSTI TTQDSLHTVL AYGEWPTYLS DIDATSVDKP THPETSADRF YTLDSVEWQV GSHGWWWKLP DALKDMGVFG Q NMYYHSMG ...String: GAQVSRQNNG THENGVTASN GSVIKYFNIN YYKDSASSGL SRQDFSQDPS KFTQPLVDTL TNPALMSPSV EACGYSDRLK QITIGNSTI TTQDSLHTVL AYGEWPTYLS DIDATSVDKP THPETSADRF YTLDSVEWQV GSHGWWWKLP DALKDMGVFG Q NMYYHSMG RSGFIIHTQC NATKFHSGAL IVAVIPEHQL AYVGGVKVNV GYDHTHPGQS GHQIRGPSQS NDRSGGKPDE DP LFNCNGT LLGNITIFPH QIINLRTNNS STIVVPYINC VPMDNMLKHN NLSLVIIPLV PLRPGSSGIN SVPITVTIAP YKS EFSGAM EAQRQ UniProtKB: Genome polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Model: Ultra thin Lacey carbon / Material: COPPER / Mesh: 400 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: LACEY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: CARBON / Support film - #1 - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.02 kPa | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 80 % / Chamber temperature: 298 K / Instrument: GATAN CRYOPLUNGE 3 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 3-70 / Number grids imaged: 3 / Number real images: 3614 / Average exposure time: 14.0 sec. / Average electron dose: 25.7 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Calibrated defocus max: 3.5 µm / Calibrated defocus min: 0.7000000000000001 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 14000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Correlation coefficient |
|---|---|
| Output model |  PDB-5jzg: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)