+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7dsn | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Overall structure of the LAT1-4F2hc bound with JX-119 | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / LAT1-4F2hc | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationL-tryptophan transmembrane transport / positive regulation of L-leucine import across plasma membrane / L-tryptophan transmembrane transporter activity / cellular response to L-arginine / apical pole of neuron / tyrosine transport / L-histidine transport / amino acid transport complex / L-leucine import across plasma membrane / L-alanine transmembrane transporter activity ...L-tryptophan transmembrane transport / positive regulation of L-leucine import across plasma membrane / L-tryptophan transmembrane transporter activity / cellular response to L-arginine / apical pole of neuron / tyrosine transport / L-histidine transport / amino acid transport complex / L-leucine import across plasma membrane / L-alanine transmembrane transporter activity / L-alanine import across plasma membrane / Defective SLC7A7 causes lysinuric protein intolerance (LPI) / aromatic amino acid transmembrane transporter activity / phenylalanine transport / methionine transport / L-leucine transmembrane transporter activity / thyroid hormone transmembrane transporter activity / isoleucine transport / valine transport / amino acid transmembrane transport / proline transport / L-amino acid transmembrane transporter activity / alanine transport / L-leucine transport / thyroid hormone transport / negative regulation of vascular associated smooth muscle cell apoptotic process / neutral amino acid transport / amino acid import across plasma membrane / positive regulation of cytokine production involved in immune response / external side of apical plasma membrane / neutral L-amino acid transmembrane transporter activity / Tryptophan catabolism / exogenous protein binding / Amino acid transport across the plasma membrane / amino acid transmembrane transporter activity / anchoring junction / antiporter activity / Basigin interactions / response to muscle activity / microvillus membrane / positive regulation of interleukin-4 production / response to exogenous dsRNA / positive regulation of interleukin-17 production / amino acid transport / tryptophan transport / positive regulation of glial cell proliferation / response to hyperoxia / xenobiotic transport / transport across blood-brain barrier / cellular response to glucose starvation / liver regeneration / negative regulation of autophagy / basal plasma membrane / peptide antigen binding / positive regulation of type II interferon production / calcium ion transport / melanosome / double-stranded RNA binding / virus receptor activity / cellular response to lipopolysaccharide / basolateral plasma membrane / carbohydrate metabolic process / apical plasma membrane / cadherin binding / protein heterodimerization activity / intracellular membrane-bounded organelle / negative regulation of gene expression / lysosomal membrane / synapse / symbiont entry into host cell / cell surface / protein homodimerization activity / RNA binding / extracellular exosome / nucleoplasm / membrane / plasma membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.1 Å | ||||||||||||
 Authors Authors | Yan, R.H. / Li, Y.N. / Zhang, Y.Y. / Zhong, X.Y. / Zhou, Q. | ||||||||||||
| Funding support |  China, 3items China, 3items
| ||||||||||||
 Citation Citation |  Journal: Cell Discov / Year: 2021 Journal: Cell Discov / Year: 2021Title: Mechanism of substrate transport and inhibition of the human LAT1-4F2hc amino acid transporter. Authors: Renhong Yan / Yaning Li / Jennifer Müller / Yuanyuan Zhang / Simon Singer / Lu Xia / Xinyue Zhong / Jürg Gertsch / Karl-Heinz Altmann / Qiang Zhou /   Abstract: LAT1 (SLC7A5) is one of the representative light chain proteins of heteromeric amino acid transporters, forming a heterodimer with its heavy chain partner 4F2hc (SLC3A2). LAT1 is overexpressed in ...LAT1 (SLC7A5) is one of the representative light chain proteins of heteromeric amino acid transporters, forming a heterodimer with its heavy chain partner 4F2hc (SLC3A2). LAT1 is overexpressed in many types of tumors and mediates the transfer of drugs and hormones across the blood-brain barrier. Thus, LAT1 is considered as a drug target for cancer treatment and may be exploited for drug delivery into the brain. Here, we synthesized three potent inhibitors of human LAT1, which inhibit transport of leucine with IC values between 100 and 250 nM, and solved the cryo-EM structures of the corresponding LAT1-4F2hc complexes with these inhibitors bound at resolution of up to 2.7 or 2.8 Å. The protein assumes an outward-facing occluded conformation, with the inhibitors bound in the classical substrate binding pocket, but with their tails wedged between the substrate binding site and TM10 of LAT1. We also solved the complex structure of LAT1-4F2hc with 3,5-diiodo-L-tyrosine (Diiodo-Tyr) at 3.4 Å overall resolution, which revealed a different inhibition mechanism and might represent an intermediate conformation between the outward-facing occluded state mentioned above and the outward-open state. To our knowledge, this is the first time that the outward-facing conformation is revealed for the HAT family. Our results unveil more important insights into the working mechanisms of HATs and provide a structural basis for future drug design. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7dsn.cif.gz 7dsn.cif.gz | 189.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7dsn.ent.gz pdb7dsn.ent.gz | 141.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7dsn.json.gz 7dsn.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ds/7dsn https://data.pdbj.org/pub/pdb/validation_reports/ds/7dsn ftp://data.pdbj.org/pub/pdb/validation_reports/ds/7dsn ftp://data.pdbj.org/pub/pdb/validation_reports/ds/7dsn | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  30839MC  7dskC  7dslC  7dsqC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 2 types, 2 molecules AB
| #1: Protein | Mass: 70160.828 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SLC3A2, hCG_2016598 / Production host: Homo sapiens (human) / Gene: SLC3A2, hCG_2016598 / Production host:  Homo sapiens (human) / References: UniProt: J3KPF3 Homo sapiens (human) / References: UniProt: J3KPF3 |
|---|---|
| #2: Protein | Mass: 57186.895 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SLC7A5, CD98LC, LAT1, MPE16 / Production host: Homo sapiens (human) / Gene: SLC7A5, CD98LC, LAT1, MPE16 / Production host:  Homo sapiens (human) / References: UniProt: Q01650 Homo sapiens (human) / References: UniProt: Q01650 |
-Sugars , 1 types, 4 molecules
| #3: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source |
|---|
-Non-polymers , 4 types, 21 molecules 
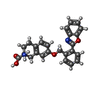





| #4: Chemical | ChemComp-3PH / | ||
|---|---|---|---|
| #5: Chemical | ChemComp-HO9 / ( | ||
| #6: Chemical | | #7: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: LAT1-4F2hc bound with JX-119 / Type: COMPLEX / Entity ID: #1-#2 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 8 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
- Processing
Processing
| EM software | Name: RELION / Version: 3.0.6 / Category: 3D reconstruction |
|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
| 3D reconstruction | Resolution: 3.1 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 641590 / Symmetry type: POINT |
 Movie
Movie Controller
Controller




















 PDBj
PDBj






