[English] 日本語
 Yorodumi
Yorodumi- PDB-7vwh: human peroxisome proliferator-activated receptor (PPAR) delta lig... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7vwh | ||||||
|---|---|---|---|---|---|---|---|
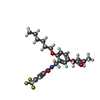
| Title | human peroxisome proliferator-activated receptor (PPAR) delta ligand binding domain in complex with a synthetic agonist JKPL39 | ||||||
 Components Components | Peroxisome proliferator-activated receptor delta | ||||||
 Keywords Keywords | TRANSCRIPTION / NUCLEAR RECEPTOR / PROTEIN-LIGAND COMPLEX / PPAR | ||||||
| Function / homology |  Function and homology information Function and homology informationkeratinocyte migration / linoleic acid binding / positive regulation of skeletal muscle tissue regeneration / fat cell proliferation / positive regulation of epidermis development / axon ensheathment / positive regulation of fat cell proliferation / regulation of skeletal muscle satellite cell proliferation / D-glucose transmembrane transport / negative regulation of smooth muscle cell migration ...keratinocyte migration / linoleic acid binding / positive regulation of skeletal muscle tissue regeneration / fat cell proliferation / positive regulation of epidermis development / axon ensheathment / positive regulation of fat cell proliferation / regulation of skeletal muscle satellite cell proliferation / D-glucose transmembrane transport / negative regulation of smooth muscle cell migration / proteoglycan metabolic process / fatty acid catabolic process / negative regulation of collagen biosynthetic process / positive regulation of myoblast proliferation / positive regulation of fatty acid oxidation / phospholipid biosynthetic process / Carnitine shuttle / negative regulation of myoblast differentiation / response to vitamin A / Signaling by Retinoic Acid / positive regulation of fatty acid metabolic process / fatty acid beta-oxidation / cell-substrate adhesion / negative regulation of cholesterol storage / decidualization / nuclear steroid receptor activity / keratinocyte proliferation / adipose tissue development / NF-kappaB binding / positive regulation of fat cell differentiation / positive regulation of insulin secretion involved in cellular response to glucose stimulus / fatty acid transport / response to glucose / cellular response to nutrient levels / cholesterol metabolic process / energy homeostasis / intracellular receptor signaling pathway / hormone-mediated signaling pathway / embryo implantation / negative regulation of miRNA transcription / response to activity / generation of precursor metabolites and energy / phosphatidylinositol 3-kinase/protein kinase B signal transduction / apoptotic signaling pathway / negative regulation of smooth muscle cell proliferation / wound healing / fatty acid metabolic process / negative regulation of cell growth / Nuclear Receptor transcription pathway / DNA-binding transcription repressor activity, RNA polymerase II-specific / lipid metabolic process / negative regulation of inflammatory response / vasodilation / transcription coactivator binding / nuclear receptor activity / glucose metabolic process / negative regulation of epithelial cell proliferation / sequence-specific double-stranded DNA binding / heart development / cellular response to lipopolysaccharide / cellular response to hypoxia / DNA-binding transcription factor activity, RNA polymerase II-specific / cell differentiation / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / cell population proliferation / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity / negative regulation of DNA-templated transcription / apoptotic process / positive regulation of gene expression / regulation of transcription by RNA polymerase II / lipid binding / negative regulation of apoptotic process / chromatin / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / DNA binding / zinc ion binding / nucleoplasm / nucleus Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Oyama, T. / Miyachi, H. | ||||||
| Funding support |  Japan, 1items Japan, 1items
| ||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.F / Year: 2022 Journal: Acta Crystallogr.,Sect.F / Year: 2022Title: Crystal structures of the ligand-binding domain of human peroxisome proliferator-activated receptor delta in complexes with phenylpropanoic acid derivatives and a pyridine carboxylic acid derivative. Authors: Oyama, T. / Takiguchi, K. / Miyachi, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7vwh.cif.gz 7vwh.cif.gz | 147.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7vwh.ent.gz pdb7vwh.ent.gz | 92.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7vwh.json.gz 7vwh.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/vw/7vwh https://data.pdbj.org/pub/pdb/validation_reports/vw/7vwh ftp://data.pdbj.org/pub/pdb/validation_reports/vw/7vwh ftp://data.pdbj.org/pub/pdb/validation_reports/vw/7vwh | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7vweC  7vwfC  7vwgC  2znpS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
|
- Components
Components
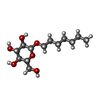
| #1: Protein | Mass: 31527.648 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PPARD, NR1C2, PPARB / Production host: Homo sapiens (human) / Gene: PPARD, NR1C2, PPARB / Production host:  #2: Chemical | #3: Sugar | #4: Water | ChemComp-HOH / | Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.82 Å3/Da / Density % sol: 56.43 % Description: THE ENTRY CONTAINS FRIEDEL PAIRS IN I/F_PLUS/MINUS COLUMNS. |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 9.5 Details: 14% PEG8000, 200MM KCL, 40MM BISTRIS ETHANE, 6% PROPANEDIOL, 0.5% N-HEPTYL-BETA-D-GLUCOPYRANOSIDE, 1MM EDTA, 1MM CACL2 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Photon Factory Photon Factory  / Beamline: AR-NE3A / Wavelength: 1 Å / Beamline: AR-NE3A / Wavelength: 1 Å |
| Detector | Type: DECTRIS PILATUS 2M-F / Detector: PIXEL / Date: Jun 8, 2015 |
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→50 Å / Num. obs: 75036 / % possible obs: 98.7 % / Redundancy: 3.3 % / Biso Wilson estimate: 32.55 Å2 / CC1/2: 0.999 / Rmerge(I) obs: 0.052 / Rpim(I) all: 0.034 / Rrim(I) all: 0.062 / Net I/σ(I): 14.3 |
| Reflection shell | Resolution: 2.1→2.16 Å / Redundancy: 3.4 % / Rmerge(I) obs: 0.431 / Mean I/σ(I) obs: 3 / Num. unique obs: 3313 / CC1/2: 0.882 / Rpim(I) all: 0.276 / Rrim(I) all: 0.513 / % possible all: 99.7 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2znp Resolution: 2.1→42.6 Å / SU ML: 0.2399 / Cross valid method: FREE R-VALUE / σ(F): 0.02 / Phase error: 27.9209 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 40.12 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→42.6 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj