+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7018 | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human apo-TRPML3 channel at pH 7.4 | ||||||||||||||||||||||||||||||
 Map data Map data | |||||||||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||||||||
 Keywords Keywords | ion channel / TRP channel / lysosomal / TRANSPORT PROTEIN | ||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationNAADP-sensitive calcium-release channel activity / inner ear auditory receptor cell differentiation / monoatomic anion channel activity / TRP channels / sodium channel activity / autophagosome membrane / potassium channel activity / locomotory behavior / calcium ion transmembrane transport / calcium channel activity ...NAADP-sensitive calcium-release channel activity / inner ear auditory receptor cell differentiation / monoatomic anion channel activity / TRP channels / sodium channel activity / autophagosome membrane / potassium channel activity / locomotory behavior / calcium ion transmembrane transport / calcium channel activity / late endosome membrane / early endosome membrane / lysosomal membrane / lipid binding / plasma membrane Similarity search - Function | ||||||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.06 Å | ||||||||||||||||||||||||||||||
 Authors Authors | Zhou X / Li M | ||||||||||||||||||||||||||||||
| Funding support |  China, China,  United States, 9 items United States, 9 items
| ||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2017 Journal: Nat Struct Mol Biol / Year: 2017Title: Cryo-EM structures of the human endolysosomal TRPML3 channel in three distinct states. Authors: Xiaoyuan Zhou / Minghui Li / Deyuan Su / Qi Jia / Huan Li / Xueming Li / Jian Yang /   Abstract: TRPML3 channels are mainly localized to endolysosomes and play a critical role in the endocytic pathway. Their dysfunction causes deafness and pigmentation defects in mice. TRPML3 activity is ...TRPML3 channels are mainly localized to endolysosomes and play a critical role in the endocytic pathway. Their dysfunction causes deafness and pigmentation defects in mice. TRPML3 activity is inhibited by low endolysosomal pH. Here we present cryo-electron microscopy (cryo-EM) structures of human TRPML3 in the closed, agonist-activated, and low-pH-inhibited states, with resolutions of 4.06, 3.62, and 4.65 Å, respectively. The agonist ML-SA1 lodges between S5 and S6 and opens an S6 gate. A polycystin-mucolipin domain (PMD) forms a luminal cap. S1 extends into this cap, forming a 'gating rod' that connects directly to a luminal pore loop, which undergoes dramatic conformational changes in response to low pH. S2 extends intracellularly and interacts with several intracellular regions to form a 'gating knob'. These unique structural features, combined with the results of electrophysiological studies, indicate a new mechanism by which luminal pH and other physiological modulators such as PIP regulate TRPML3 by changing S1 and S2 conformations. | ||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7018.map.gz emd_7018.map.gz | 2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7018-v30.xml emd-7018-v30.xml emd-7018.xml emd-7018.xml | 15.4 KB 15.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_7018.png emd_7018.png | 89.3 KB | ||
| Filedesc metadata |  emd-7018.cif.gz emd-7018.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7018 http://ftp.pdbj.org/pub/emdb/structures/EMD-7018 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7018 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7018 | HTTPS FTP |
-Related structure data
| Related structure data |  6ayeMC  7019C  7020C  6ayfC  6aygC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_7018.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7018.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
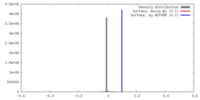
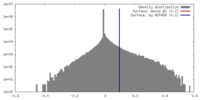
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : human TRPML3 apo channel at pH 7.4
| Entire | Name: human TRPML3 apo channel at pH 7.4 |
|---|---|
| Components |
|
-Supramolecule #1: human TRPML3 apo channel at pH 7.4
| Supramolecule | Name: human TRPML3 apo channel at pH 7.4 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Mucolipin-3
| Macromolecule | Name: Mucolipin-3 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 64.625785 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: GGGGSMADPE VVVSSCSSHE EENRCNFNQQ TSPSEELLLE DQMRRKLKFF FMNPCEKFWA RGRKPWKLAI QILKIAMVTI QLVLFGLSN QMVVAFKEEN TIAFKHLFLK GYMDRMDDTY AVYTQSDVYD QLIFAVNQYL QLYNVSVGNH AYENKGTKQS A MAICQHFY ...String: GGGGSMADPE VVVSSCSSHE EENRCNFNQQ TSPSEELLLE DQMRRKLKFF FMNPCEKFWA RGRKPWKLAI QILKIAMVTI QLVLFGLSN QMVVAFKEEN TIAFKHLFLK GYMDRMDDTY AVYTQSDVYD QLIFAVNQYL QLYNVSVGNH AYENKGTKQS A MAICQHFY KRGNIYPGND TFDIDPEIET ECFFVEPDEP FHIGTPAENK LNLTLDFHRL LTVELQFKLK AINLQTVRHQ EL PDCYDFT LTITFDNKAH SGRIKISLDN DISIRECKDW HVSGSIQKNT HYMMIFDAFV ILTCLVSLIL CIRSVIRGLQ LQQ EFVNFF LLHYKKEVSV SDQMEFVNGW YIMIIISDIL TIIGSILKME IQAKSLTSYD VCSILLGTST MLVWLGVIRY LGFF AKYNL LILTLQAALP NVIRFCCCAA MIYLGYCFCG WIVLGPYHDK FRSLNMVSEC LFSLINGDDM FATFAKMQQK SYLVW LFSR IYLYSFISLF IYMILSLFIA LITDTYETIK QYQQDGFPET ELRTFISECK DLPNSGKYRL EDDPPVSLFC CCKK UniProtKB: Mucolipin-3 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.9 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: The pH of the buffer was adjusted to 7.4 by NaOH. | |||||||||
| Grid | Model: Quantifoil holey carbon grid R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV Details: waiting for 3 seconds before blotting for 4 seconds(double-sided, blot force 1),then the grid was immediately plunged into liquid ethane cooled by liquid-nitrogen. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 7676 pixel / Digitization - Dimensions - Height: 7420 pixel / Digitization - Frames/image: 1-32 / Average exposure time: 8.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)