+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5mg9 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Putative Ancestral Mamba toxin 1 (AncTx1-W28R/I38S) | ||||||
 Components Components | AncTx1-W28R/I38S | ||||||
 Keywords Keywords | TOXIN / Ancestral mamba snake toxin / three-finger fold / Ancestral toxin resurrection and engineering / aminergic toxin | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  Dendroaspis angusticeps (eastern green mamba) Dendroaspis angusticeps (eastern green mamba) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.801 Å MOLECULAR REPLACEMENT / Resolution: 1.801 Å | ||||||
 Authors Authors | Stura, E.A. / Tepshi, L. / Blanchet, G. / Mourier, G. / Servent, D. | ||||||
 Citation Citation |  Journal: Sci Rep / Year: 2017 Journal: Sci Rep / Year: 2017Title: Ancestral protein resurrection and engineering opportunities of the mamba aminergic toxins. Authors: Blanchet, G. / Alili, D. / Protte, A. / Upert, G. / Gilles, N. / Tepshi, L. / Stura, E.A. / Mourier, G. / Servent, D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5mg9.cif.gz 5mg9.cif.gz | 45.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5mg9.ent.gz pdb5mg9.ent.gz | 31.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5mg9.json.gz 5mg9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5mg9_validation.pdf.gz 5mg9_validation.pdf.gz | 468.8 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5mg9_full_validation.pdf.gz 5mg9_full_validation.pdf.gz | 469.4 KB | Display | |
| Data in XML |  5mg9_validation.xml.gz 5mg9_validation.xml.gz | 6.6 KB | Display | |
| Data in CIF |  5mg9_validation.cif.gz 5mg9_validation.cif.gz | 8.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/mg/5mg9 https://data.pdbj.org/pub/pdb/validation_reports/mg/5mg9 ftp://data.pdbj.org/pub/pdb/validation_reports/mg/5mg9 ftp://data.pdbj.org/pub/pdb/validation_reports/mg/5mg9 | HTTPS FTP |
-Related structure data
| Related structure data |  4iyeS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 7255.345 Da / Num. of mol.: 1 / Source method: obtained synthetically Details: Putative ancetral mamba toxin, Residues 1 to 6 (LEU THR CYS VAL LYS SER ) match to UNP Q8QGR0 NXM11_DENAN 22 to 28, Residues 7 to 11 (LYS SER ILE PHE GLY ) match to UNP P85092 TXAD1_DENAN 7 ...Details: Putative ancetral mamba toxin, Residues 1 to 6 (LEU THR CYS VAL LYS SER ) match to UNP Q8QGR0 NXM11_DENAN 22 to 28, Residues 7 to 11 (LYS SER ILE PHE GLY ) match to UNP P85092 TXAD1_DENAN 7 to 11, Residues 12 to 17 (VAL THR THR GLU ASP CYS ) match to UNP P18328 TXM2_DENAN 12 to 17, Residues 18 to 26 (PRO ASP GLY GLN ASN LEU CYS PHE LYS ) match to UNP P81030 TXM1_DENAN 18 to 26, Residues 27 to 31 (ARG ARG HIS TYR ILE ) match to UNP P86419 3SI1B_DENAN 27 to 31, Residues 32 to 34 (VAL PRO LYS ) match to UNP P85092 TXAD1_DENAN 32 to 34, Residues 35 to 37 (MET TYR ASP ) match to UNP Q8QGR0 NXM11_DENAN 56 to 58, Residues 38 to 48 (SER THR ARG GLY CYS ALA ALA THR CYS PRO ILE ) match to UNP P85092 TXAD1_DENAN 38 to 48, Residues 49 to 50 (ALA GLU ) match to UNP Q8QGR0 NXM11_DENAN 61 to 62, Residues 51 to 54 (ASN ARG ASP VAL ) match to UNP P82462 3SUC1_NAJKA 51 to 54, Residues 55 to 58 (ILE HIS CYS CYS ) match to UNP P85092 TXAD1_DENAN 55 to 58, Residues 59 to 62 (GLY THR ASP LYS ) match to UNP P18328 TXM2_DENAN 59 to 62, Residues 63 to 65 (CYS ASN GLU ) match to UNP P85092 TXAD1_DENAN 63 to 65, Source: (synth.)  Dendroaspis angusticeps (eastern green mamba) Dendroaspis angusticeps (eastern green mamba)References: UniProt: P85092*PLUS |
|---|
-Non-polymers , 5 types, 94 molecules 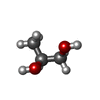


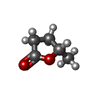





| #2: Chemical | ChemComp-PGO / | ||||
|---|---|---|---|---|---|
| #3: Chemical | ChemComp-EDO / | ||||
| #4: Chemical | ChemComp-SO4 / #5: Chemical | ChemComp-YVR / | #6: Water | ChemComp-HOH / | |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.25 Å3/Da / Density % sol: 45.44 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 5.5 Details: Protein: lyophilized synthetic toxin at 5 mg/mL in 1 M Na Acetate pH 5.5. Precipitant: 1.9 M ammonium sulfate, 4 % MPD, 2 % 1,4-dioxane, 2 % gamma-valerolactone, 0.132 M sodium citrate, pH 5. ...Details: Protein: lyophilized synthetic toxin at 5 mg/mL in 1 M Na Acetate pH 5.5. Precipitant: 1.9 M ammonium sulfate, 4 % MPD, 2 % 1,4-dioxane, 2 % gamma-valerolactone, 0.132 M sodium citrate, pH 5.5. Cryoprotectant: 80% saturated lithium sulfate, 10% Dioxane, 10% gamma-valerolactone PH range: 5.5 / Temp details: Cooled incubator |
-Data collection
| Diffraction | Mean temperature: 100 K / Ambient temp details: cryostream |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: MASSIF-1 / Wavelength: 0.965 Å / Beamline: MASSIF-1 / Wavelength: 0.965 Å |
| Detector | Type: DECTRIS PILATUS 2M / Detector: PIXEL / Date: Jan 29, 2015 Details: Compound Refractive Lens Fully automatic data collection |
| Radiation | Monochromator: diamond beam splitter / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.965 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→35.556 Å / Num. obs: 11687 / % possible obs: 100 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -3 / Redundancy: 8.3 % / CC1/2: 0.999 / Rmerge(I) obs: 0.111 / Rsym value: 0.104 / Net I/σ(I): 12.24 |
| Reflection shell | Resolution: 1.8→1.91 Å / Redundancy: 8.2 % / Rmerge(I) obs: 1.53 / Mean I/σ(I) obs: 1.16 / CC1/2: 0.719 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4IYE Resolution: 1.801→35.556 Å / SU ML: 0.29 / Cross valid method: THROUGHOUT / σ(F): 1.34 / Phase error: 30.62 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.801→35.556 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 20.7852 Å / Origin y: -8.891 Å / Origin z: -8.7359 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller











 PDBj
PDBj





