[English] 日本語
 Yorodumi
Yorodumi- PDB-4wdx: 17beta-HSD5 in complex with [4-(2-hydroxyethyl)piperidin-1-yl](5-... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4wdx | ||||||
|---|---|---|---|---|---|---|---|
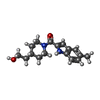
| Title | 17beta-HSD5 in complex with [4-(2-hydroxyethyl)piperidin-1-yl](5-methyl-1H-indol-2-yl)methanone | ||||||
 Components Components | Aldo-keto reductase family 1 member C3 | ||||||
 Keywords Keywords | OXIDOREDUCTASE/OXIDOREDUCTASE INHIBITOR / aldo-keto reductase inhibitor / OXIDOREDUCTASE-OXIDOREDUCTASE INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationprostaglandin-F synthase / testosterone 17beta-dehydrogenase (NADP+) / prostaglandin D2 11-ketoreductase activity / ketoreductase activity / prostaglandin F synthase activity / cellular response to prostaglandin stimulus / cellular response to corticosteroid stimulus / macromolecule metabolic process / 15-hydroxyprostaglandin-D dehydrogenase (NADP+) activity / 3beta(or 20alpha)-hydroxysteroid dehydrogenase ...prostaglandin-F synthase / testosterone 17beta-dehydrogenase (NADP+) / prostaglandin D2 11-ketoreductase activity / ketoreductase activity / prostaglandin F synthase activity / cellular response to prostaglandin stimulus / cellular response to corticosteroid stimulus / macromolecule metabolic process / 15-hydroxyprostaglandin-D dehydrogenase (NADP+) activity / 3beta(or 20alpha)-hydroxysteroid dehydrogenase / negative regulation of retinoic acid biosynthetic process / 5-alpha-androstane-3-beta,17-beta-diol dehydrogenase (NADP+) activity / Delta4-3-oxosteroid 5beta-reductase activity / farnesol catabolic process / geranylgeranyl reductase activity / 3alpha-hydroxysteroid 3-dehydrogenase / cellular response to jasmonic acid stimulus / 3alpha(17beta)-hydroxysteroid dehydrogenase (NAD+) / prostanoid biosynthetic process / testosterone dehydrogenase (NADP+) activity / androsterone dehydrogenase [NAD(P)+] activity / ketosteroid monooxygenase activity / regulation of testosterone biosynthetic process / RA biosynthesis pathway / testosterone biosynthetic process / : / 3alpha(or 20beta)-hydroxysteroid dehydrogenase / androstan-3-alpha,17-beta-diol dehydrogenase (NAD+) activity / Synthesis of bile acids and bile salts via 24-hydroxycholesterol / testosterone dehydrogenase (NAD+) activity / regulation of retinoic acid receptor signaling pathway / cellular response to prostaglandin D stimulus / progesterone metabolic process / 17beta-estradiol 17-dehydrogenase / retinal metabolic process / Synthesis of Prostaglandins (PG) and Thromboxanes (TX) / estradiol 17-beta-dehydrogenase [NAD(P)+] activity / all-trans-retinol dehydrogenase (NAD+) activity / : / prostaglandin H2 endoperoxidase reductase activity / all-trans-retinol dehydrogenase (NADP+) activity / Oxidoreductases; Acting on the CH-OH group of donors; With NAD+ or NADP+ as acceptor / bile acid binding / daunorubicin metabolic process / doxorubicin metabolic process / retinal dehydrogenase (NAD+) activity / aldose reductase (NADPH) activity / oxidoreductase activity, acting on NAD(P)H, quinone or similar compound as acceptor / prostaglandin metabolic process / renal absorption / steroid metabolic process / positive regulation of endothelial cell apoptotic process / Synthesis of bile acids and bile salts via 27-hydroxycholesterol / Synthesis of bile acids and bile salts via 7alpha-hydroxycholesterol / retinoid metabolic process / Retinoid metabolism and transport / keratinocyte differentiation / response to nutrient / cellular response to calcium ion / cellular response to starvation / male gonad development / positive regulation of reactive oxygen species metabolic process / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / G protein-coupled receptor signaling pathway / positive regulation of cell population proliferation / extracellular exosome / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.64 Å X-RAY DIFFRACTION / Resolution: 1.64 Å | ||||||
 Authors Authors | Amano, Y. / Yamaguchi, T. | ||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 2015 Journal: Acta Crystallogr.,Sect.D / Year: 2015Title: Structures of complexes of type 5 17 beta-hydroxysteroid dehydrogenase with structurally diverse inhibitors: insights into the conformational changes upon inhibitor binding. Authors: Amano, Y. / Yamaguchi, T. / Niimi, T. / Sakashita, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4wdx.cif.gz 4wdx.cif.gz | 141.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4wdx.ent.gz pdb4wdx.ent.gz | 109.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4wdx.json.gz 4wdx.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wd/4wdx https://data.pdbj.org/pub/pdb/validation_reports/wd/4wdx ftp://data.pdbj.org/pub/pdb/validation_reports/wd/4wdx ftp://data.pdbj.org/pub/pdb/validation_reports/wd/4wdx | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4wdtC  4wduC  4wdwC  4xvdC  4xveC  4wds  4wdv C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 37967.355 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: AKR1C3 / Production host: Homo sapiens (human) / Gene: AKR1C3 / Production host:  References: UniProt: P42330, testosterone 17beta-dehydrogenase (NADP+) #2: Chemical | #3: Chemical | #4: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.19 Å3/Da / Density % sol: 43.71 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: 0.1 M HEPES, 0.2M ammonium dihydrogen phosphate, 20-25% (w/v) PEG3350 |
-Data collection
| Diffraction | Mean temperature: 90 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU FR-E+ SUPERBRIGHT / Wavelength: 1.5418 Å |
| Detector | Type: RIGAKU RAXIS VII / Detector: IMAGE PLATE / Date: Jul 16, 2008 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.64→59.51 Å / Num. obs: 76592 / % possible obs: 95.7 % / Redundancy: 3.6 % / Net I/σ(I): 6.7 |
- Processing
Processing
| Software | Name: REFMAC / Version: 5.7.0029 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.64→14.27 Å / Cor.coef. Fo:Fc: 0.959 / Cor.coef. Fo:Fc free: 0.945 / SU B: 2.346 / SU ML: 0.079 / Cross valid method: THROUGHOUT / ESU R: 0.115 / ESU R Free: 0.113 / Stereochemistry target values: MAXIMUM LIKELIHOOD
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 27.64 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 1.64→14.27 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj