[English] 日本語
 Yorodumi
Yorodumi- PDB-4d3u: Structure of Bacillus subtilis Nitric Oxide Synthase H128S in com... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4d3u | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Bacillus subtilis Nitric Oxide Synthase H128S in complex with N-{3-[(1S)-2-(3-{(Z)-[amino(thiophen-2-yl)methylidene]amino}phenoxy)-1-hydroxyethyl]phenyl}thiophene-2-carboximidamide | |||||||||
 Components Components | NITRIC OXIDE SYNTHASE OXYGENASE | |||||||||
 Keywords Keywords | OXIDOREDUCTASE / INHIBITOR | |||||||||
| Function / homology |  Function and homology information Function and homology informationnitric-oxide synthase (flavodoxin) / nitric-oxide synthase activity / nitric oxide biosynthetic process / heme binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.981 Å MOLECULAR REPLACEMENT / Resolution: 1.981 Å | |||||||||
 Authors Authors | Holden, J.K. / Poulos, T.L. | |||||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2015 Journal: J.Med.Chem. / Year: 2015Title: Structure-Based Design of Bacterial Nitric Oxide Synthase Inhibitors. Authors: Holden, J.K. / Kang, S. / Hollingsworth, S.A. / Li, H. / Lim, N. / Chen, S. / Huang, H. / Xue, F. / Tang, W. / Silverman, R.B. / Poulos, T.L. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4d3u.cif.gz 4d3u.cif.gz | 175.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4d3u.ent.gz pdb4d3u.ent.gz | 137.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4d3u.json.gz 4d3u.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4d3u_validation.pdf.gz 4d3u_validation.pdf.gz | 1.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4d3u_full_validation.pdf.gz 4d3u_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  4d3u_validation.xml.gz 4d3u_validation.xml.gz | 19.8 KB | Display | |
| Data in CIF |  4d3u_validation.cif.gz 4d3u_validation.cif.gz | 26.9 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/d3/4d3u https://data.pdbj.org/pub/pdb/validation_reports/d3/4d3u ftp://data.pdbj.org/pub/pdb/validation_reports/d3/4d3u ftp://data.pdbj.org/pub/pdb/validation_reports/d3/4d3u | HTTPS FTP |
-Related structure data
| Related structure data |  4d3iC  4d3jC  4d3kC  4d3mC  4d3nC  4d3oC  4d3tC  4d3vC  4lwaS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 42018.293 Da / Num. of mol.: 1 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Production host:  |
|---|
-Non-polymers , 6 types, 177 molecules 

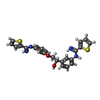







| #2: Chemical | ChemComp-HEM / | ||||
|---|---|---|---|---|---|
| #3: Chemical | ChemComp-CL / | ||||
| #4: Chemical | ChemComp-RFQ / | ||||
| #5: Chemical | | #6: Chemical | #7: Water | ChemComp-HOH / | |
-Details
| Has protein modification | N | ||
|---|---|---|---|
| Nonpolymer details | (S)-N-(3-(1-HYDROXY-2-(3-(THIOPHENE-2-CARBOXIMID| Sequence details | E25A, E26A,E316A AND H128S INTRODUCED | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.83 Å3/Da / Density % sol: 56.53 % / Description: NONE |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7.6 Details: 60 MM BIS-TRIS METHANE, 40 MM CITRIC ACID, 20% PEG3350, 1.9% 1-PROPANOL, PH 7.6, VAPOR DIFFUSION, HANGING DROP, TEMPERATURE 298K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRL SSRL  / Beamline: BL11-1 / Wavelength: 0.97891 / Beamline: BL11-1 / Wavelength: 0.97891 |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Nov 30, 2013 / Details: MIRRORS |
| Radiation | Monochromator: SI(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97891 Å / Relative weight: 1 |
| Reflection | Resolution: 1.98→37.65 Å / Num. obs: 33624 / % possible obs: 99.2 % / Observed criterion σ(I): -3 / Redundancy: 5.1 % / Biso Wilson estimate: 36.44 Å2 / Rmerge(I) obs: 0.04 / Net I/σ(I): 20.1 |
| Reflection shell | Resolution: 1.98→2.03 Å / Redundancy: 4.9 % / Rmerge(I) obs: 1.3 / Mean I/σ(I) obs: 1 / % possible all: 99.2 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 4LWA Resolution: 1.981→29.417 Å / SU ML: 0.22 / σ(F): 1.35 / Phase error: 24.65 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.981→29.417 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 5.5875 Å / Origin y: 19.6463 Å / Origin z: 22.3769 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: ALL |
 Movie
Movie Controller
Controller






















 PDBj
PDBj





