+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4970 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the core TFIIH-XPA-DNA complex | |||||||||||||||
 Map data Map data | Composite map produced from focused refined maps (deposited as additional EM maps). | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Complex / Helicase / Translocase / DNA repair | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnucleotide-excision repair factor 1 complex / nucleotide-excision repair involved in interstrand cross-link repair / nucleotide-excision repair, DNA damage recognition / MMXD complex / core TFIIH complex portion of holo TFIIH complex / Cytosolic iron-sulfur cluster assembly / central nervous system myelin formation / positive regulation of mitotic recombination / hair cell differentiation / hair follicle maturation ...nucleotide-excision repair factor 1 complex / nucleotide-excision repair involved in interstrand cross-link repair / nucleotide-excision repair, DNA damage recognition / MMXD complex / core TFIIH complex portion of holo TFIIH complex / Cytosolic iron-sulfur cluster assembly / central nervous system myelin formation / positive regulation of mitotic recombination / hair cell differentiation / hair follicle maturation / nucleotide-excision repair factor 3 complex / nucleotide-excision repair, preincision complex assembly / CAK-ERCC2 complex / embryonic cleavage / DNA 5'-3' helicase / UV protection / regulation of cyclin-dependent protein serine/threonine kinase activity / transcription factor TFIIH core complex / transcription factor TFIIH holo complex / G protein-coupled receptor internalization / nuclear thyroid hormone receptor binding / UV-damage excision repair / transcription preinitiation complex / RNA Polymerase I Transcription Termination / RNA polymerase II general transcription initiation factor activity / transcription factor TFIID complex / regulation of mitotic cell cycle phase transition / erythrocyte maturation / hematopoietic stem cell proliferation / RNA Pol II CTD phosphorylation and interaction with CE during HIV infection / RNA Pol II CTD phosphorylation and interaction with CE / spinal cord development / 3'-5' DNA helicase activity / Formation of the Early Elongation Complex / Formation of the HIV-1 Early Elongation Complex / bone mineralization / mRNA Capping / DNA 3'-5' helicase / HIV Transcription Initiation / RNA Polymerase II HIV Promoter Escape / Transcription of the HIV genome / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / ATPase activator activity / DNA topological change / intrinsic apoptotic signaling pathway by p53 class mediator / RNA Polymerase I Transcription Initiation / embryonic organ development / hematopoietic stem cell differentiation / positive regulation of transcription initiation by RNA polymerase II / protein localization to nucleus / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / transcription elongation by RNA polymerase I / Formation of HIV elongation complex in the absence of HIV Tat / response to UV / transcription by RNA polymerase I / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / hormone-mediated signaling pathway / transcription-coupled nucleotide-excision repair / extracellular matrix organization / RNA Polymerase II Pre-transcription Events / insulin-like growth factor receptor signaling pathway / DNA helicase activity / determination of adult lifespan / post-embryonic development / maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / TP53 Regulates Transcription of DNA Repair Genes / nucleotide-excision repair / transcription initiation at RNA polymerase II promoter / transcription elongation by RNA polymerase II / chromosome segregation / promoter-specific chromatin binding / RNA Polymerase I Promoter Escape / cellular response to gamma radiation / base-excision repair / NoRC negatively regulates rRNA expression / regulation of autophagy / response to toxic substance / multicellular organism growth / Dual Incision in GG-NER / Transcription-Coupled Nucleotide Excision Repair (TC-NER) / spindle / Formation of TC-NER Pre-Incision Complex / intrinsic apoptotic signaling pathway in response to DNA damage / Formation of Incision Complex in GG-NER / sequence-specific double-stranded DNA binding / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / intracellular protein localization / 4 iron, 4 sulfur cluster binding / response to oxidative stress / double-stranded DNA binding / 5'-3' DNA helicase activity / protein-macromolecule adaptor activity / in utero embryonic development / transcription by RNA polymerase II Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
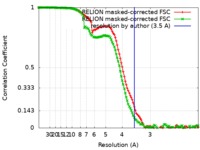
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||||||||
 Authors Authors | Kokic G / Chernev A / Tegunov D / Dienemann C / Urlaub H / Cramer P | |||||||||||||||
| Funding support |  Germany, 4 items Germany, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2019 Journal: Nat Commun / Year: 2019Title: Structural basis of TFIIH activation for nucleotide excision repair. Authors: Goran Kokic / Aleksandar Chernev / Dimitry Tegunov / Christian Dienemann / Henning Urlaub / Patrick Cramer /  Abstract: Nucleotide excision repair (NER) is the major DNA repair pathway that removes UV-induced and bulky DNA lesions. There is currently no structure of NER intermediates, which form around the large ...Nucleotide excision repair (NER) is the major DNA repair pathway that removes UV-induced and bulky DNA lesions. There is currently no structure of NER intermediates, which form around the large multisubunit transcription factor IIH (TFIIH). Here we report the cryo-EM structure of an NER intermediate containing TFIIH and the NER factor XPA. Compared to its transcription conformation, the TFIIH structure is rearranged such that its ATPase subunits XPB and XPD bind double- and single-stranded DNA, consistent with their translocase and helicase activities, respectively. XPA releases the inhibitory kinase module of TFIIH, displaces a 'plug' element from the DNA-binding pore in XPD, and together with the NER factor XPG stimulates XPD activity. Our results explain how TFIIH is switched from a transcription to a repair factor, and provide the basis for a mechanistic analysis of the NER pathway. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4970.map.gz emd_4970.map.gz | 33.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4970-v30.xml emd-4970-v30.xml emd-4970.xml emd-4970.xml | 43.2 KB 43.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4970_fsc_1.xml emd_4970_fsc_1.xml emd_4970_fsc_2.xml emd_4970_fsc_2.xml | 11.4 KB 11.4 KB | Display Display |  FSC data file FSC data file |
| Images |  emd_4970.png emd_4970.png | 98.3 KB | ||
| Masks |  emd_4970_msk_1.map emd_4970_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-4970.cif.gz emd-4970.cif.gz | 8.8 KB | ||
| Others |  emd_4970_additional_1.map.gz emd_4970_additional_1.map.gz emd_4970_additional_2.map.gz emd_4970_additional_2.map.gz emd_4970_additional_3.map.gz emd_4970_additional_3.map.gz emd_4970_additional_4.map.gz emd_4970_additional_4.map.gz emd_4970_additional_5.map.gz emd_4970_additional_5.map.gz emd_4970_additional_6.map.gz emd_4970_additional_6.map.gz emd_4970_additional_7.map.gz emd_4970_additional_7.map.gz emd_4970_additional_8.map.gz emd_4970_additional_8.map.gz emd_4970_additional_9.map.gz emd_4970_additional_9.map.gz | 112.8 MB 112.8 MB 38.7 MB 98.8 MB 98.8 MB 7.2 MB 6.4 MB 6.4 MB 5.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4970 http://ftp.pdbj.org/pub/emdb/structures/EMD-4970 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4970 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4970 | HTTPS FTP |
-Related structure data
| Related structure data |  6ro4MC  7ad8M M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4970.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4970.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map produced from focused refined maps (deposited as additional EM maps). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
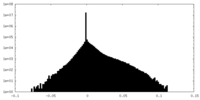
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
+Mask #1
+Additional map: Half map 1 corresponding to the composite map.
+Additional map: Half map 2 corresponding to the composite map.
+Additional map: Globally refined map.
+Additional map: Half map 1 corresponding to the globally refined map.
+Additional map: Half map 2 corresponding to the globally refined map.
+Additional map: Focused classified and refined map using a mask...
+Additional map: Focused classified and refined map using a mask...
+Additional map: Focused classified and refined map using a mask...
+Additional map: Focused classified and refined map using a mask...
- Sample components
Sample components
+Entire : Core TFIIH-XPA-DNA complex
+Supramolecule #1: Core TFIIH-XPA-DNA complex
+Supramolecule #2: TFIIH-XPA
+Supramolecule #3: DNA
+Macromolecule #1: DNA1
+Macromolecule #2: DNA2
+Macromolecule #3: General transcription and DNA repair factor IIH helicase subunit XPB
+Macromolecule #4: General transcription factor IIH subunit 5
+Macromolecule #5: TFIIH basal transcription factor complex helicase XPD subunit
+Macromolecule #6: General transcription factor IIH subunit 3
+Macromolecule #7: General transcription factor IIH subunit 2
+Macromolecule #8: General transcription factor IIH subunit 4
+Macromolecule #9: DNA repair protein complementing XP-A cells
+Macromolecule #10: IRON/SULFUR CLUSTER
+Macromolecule #11: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: GOLD / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: 4 ul of sample was applied to glow-discharged grids which were blotted for 5s and plunge-frozen in liquid ethane.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 41.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 130000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)



















































































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)