+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4571 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Elongator catalytic subcomplex Elp123 lobe | |||||||||
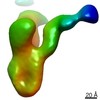
 Map data Map data | Postprocessed map of Elongator catalytic subcomplex Elp123 lobe from yeast at 3.3 A resolution. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Elongator / yeast / tRNA modification / Elp123 / TRANSLATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationtRNA uridine(34) acetyltransferase activity / tRNA carboxymethyluridine synthase / elongator holoenzyme complex / protein urmylation / tRNA wobble base 5-methoxycarbonylmethyl-2-thiouridinylation / tRNA wobble uridine modification / protein transport / regulation of translation / 4 iron, 4 sulfur cluster binding / microtubule binding ...tRNA uridine(34) acetyltransferase activity / tRNA carboxymethyluridine synthase / elongator holoenzyme complex / protein urmylation / tRNA wobble base 5-methoxycarbonylmethyl-2-thiouridinylation / tRNA wobble uridine modification / protein transport / regulation of translation / 4 iron, 4 sulfur cluster binding / microtubule binding / tRNA binding / regulation of transcription by RNA polymerase II / nucleoplasm / identical protein binding / nucleus / metal ion binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Dauden MI / Weis F | |||||||||
| Funding support |  Germany, Germany,  Poland, 2 items Poland, 2 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2019 Journal: Sci Adv / Year: 2019Title: Molecular basis of tRNA recognition by the Elongator complex. Authors: Maria I Dauden / Marcin Jaciuk / Felix Weis / Ting-Yu Lin / Carolin Kleindienst / Nour El Hana Abbassi / Heena Khatter / Rościsław Krutyhołowa / Karin D Breunig / Jan Kosinski / Christoph ...Authors: Maria I Dauden / Marcin Jaciuk / Felix Weis / Ting-Yu Lin / Carolin Kleindienst / Nour El Hana Abbassi / Heena Khatter / Rościsław Krutyhołowa / Karin D Breunig / Jan Kosinski / Christoph W Müller / Sebastian Glatt /   Abstract: The highly conserved Elongator complex modifies transfer RNAs (tRNAs) in their wobble base position, thereby regulating protein synthesis and ensuring proteome stability. The precise mechanisms of ...The highly conserved Elongator complex modifies transfer RNAs (tRNAs) in their wobble base position, thereby regulating protein synthesis and ensuring proteome stability. The precise mechanisms of tRNA recognition and its modification reaction remain elusive. Here, we show cryo-electron microscopy structures of the catalytic subcomplex of Elongator and its tRNA-bound state at resolutions of 3.3 and 4.4 Å. The structures resolve details of the catalytic site, including the substrate tRNA, the iron-sulfur cluster, and a SAM molecule, which are all validated by mutational analyses in vitro and in vivo. tRNA binding induces conformational rearrangements, which precisely position the targeted anticodon base in the active site. Our results provide the molecular basis for substrate recognition of Elongator, essential to understand its cellular function and role in neurodegenerative diseases and cancer. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4571.map.gz emd_4571.map.gz | 1.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4571-v30.xml emd-4571-v30.xml emd-4571.xml emd-4571.xml | 29.6 KB 29.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4571_fsc.xml emd_4571_fsc.xml | 13 KB | Display |  FSC data file FSC data file |
| Images |  emd_4571.png emd_4571.png | 187.7 KB | ||
| Filedesc metadata |  emd-4571.cif.gz emd-4571.cif.gz | 8.8 KB | ||
| Others |  emd_4571_additional_1.map.gz emd_4571_additional_1.map.gz emd_4571_additional_2.map.gz emd_4571_additional_2.map.gz emd_4571_half_map_1.map.gz emd_4571_half_map_1.map.gz emd_4571_half_map_2.map.gz emd_4571_half_map_2.map.gz | 2.6 MB 1.6 MB 85.7 MB 85.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4571 http://ftp.pdbj.org/pub/emdb/structures/EMD-4571 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4571 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4571 | HTTPS FTP |
-Validation report
| Summary document |  emd_4571_validation.pdf.gz emd_4571_validation.pdf.gz | 385.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_4571_full_validation.pdf.gz emd_4571_full_validation.pdf.gz | 385 KB | Display | |
| Data in XML |  emd_4571_validation.xml.gz emd_4571_validation.xml.gz | 16.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4571 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4571 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4571 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4571 | HTTPS FTP |
-Related structure data
| Related structure data |  6qk7MC  4573C  4574C  4576C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4571.map.gz / Format: CCP4 / Size: 93 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4571.map.gz / Format: CCP4 / Size: 93 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessed map of Elongator catalytic subcomplex Elp123 lobe from yeast at 3.3 A resolution. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.35 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: LocScale map of Elongator catalytic subcomplex Elp123 lobe...
| File | emd_4571_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | LocScale map of Elongator catalytic subcomplex Elp123 lobe including the dimerization domain (DD) of Elp1, used to build the atomic model of the C-terminal domains of Elp1. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: LocScale map of Elongator catalytic subcomplex Elp123 lobe...
| File | emd_4571_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | LocScale map of Elongator catalytic subcomplex Elp123 lobe used to build the atomic model. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of Elongator catalytic subcomplex Elp123 lobe from yeast.
| File | emd_4571_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of Elongator catalytic subcomplex Elp123 lobe from yeast. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map of Elongator catalytic subcomplex Elp123 lobe from yeast.
| File | emd_4571_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map of Elongator catalytic subcomplex Elp123 lobe from yeast. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Elongator catalytic subcomplex Elp123
| Entire | Name: Elongator catalytic subcomplex Elp123 |
|---|---|
| Components |
|
-Supramolecule #1: Elongator catalytic subcomplex Elp123
| Supramolecule | Name: Elongator catalytic subcomplex Elp123 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 Details: The EM map corresponds to one lobe of the Elp123 complex, that includes one copy of Elp1, Elp2 and Elp3, and the C-terminal part of a second copy of Elp1. |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 621 KDa |
-Macromolecule #1: Elongator complex protein 1
| Macromolecule | Name: Elongator complex protein 1 / type: protein_or_peptide / ID: 1 Details: Chain D corresponds to the C-terminal domain of Elp1 Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: ATCC 204508 / S288c |
| Molecular weight | Theoretical: 153.166266 KDa |
| Sequence | String: MVEHDKSGSK RQELRSNMRN LITLNKGKFK PTASTAEGDE DDLSFTLLDS VFDTLSDSIT CVLGSTDIGA IEVQQFMKDG SRNVLASFN IQTFDDKLLS FVHFADINQL VFVFEQGDII TATYDPVSLD PAETLIEIMG TIDNGIAAAQ WSYDEETLAM V TKDRNVVV ...String: MVEHDKSGSK RQELRSNMRN LITLNKGKFK PTASTAEGDE DDLSFTLLDS VFDTLSDSIT CVLGSTDIGA IEVQQFMKDG SRNVLASFN IQTFDDKLLS FVHFADINQL VFVFEQGDII TATYDPVSLD PAETLIEIMG TIDNGIAAAQ WSYDEETLAM V TKDRNVVV LSKLFEPISE YHLEVDDLKI SKHVTVGWGK KETQFRGKGA RAMEREALAS LKASGLVGNQ LRDPTMPYMV DT GDVTALD SHEITISWRG DCDYFAVSSV EEVPDEDDET KSIKRRAFRV FSREGQLDSA SEPVTGMEHQ LSWKPQGSLI ASI QRKTDL GEEDSVDVIF FERNGLRHGE FDTRLPLDEK VESVCWNSNS EALAVVLANR IQLWTSKNYH WYLKQELYAS DISY VKWHP EKDFTLMFSD AGFINIVDFA YKMAQGPTLE PFDNGTSLVV DGRTVNITPL ALANVPPPMY YRDFETPGNV LDVAC SFSN EIYAAINKDV LIFAAVPSIE EMKKGKHPSI VCEFPKSEFT SEVDSLRQVA FINDSIVGVL LDTDNLSRIA LLDIQD ITQ PTLITIVEVY DKIVLLRSDF DYNHLVYETR DGTVCQLDAE GQLMEITKFP QLVRDFRVKR VHNTSAEDDD NWSAESS EL VAFGITNNGK LFANQVLLAS AVTSLEITDS FLLFTTAQHN LQFVHLNSTD FKPLPLVEEG VEDERVRAIE RGSILVSV I PSKSSVVLQA TRGNLETIYP RIMVLAEVRK NIMAKRYKEA FIVCRTHRIN LDILHDYAPE LFIENLEVFI NQIGRVDYL NLFISCLSED DVTKTKYKET LYSGISKSFG MEPAPLTEMQ IYMKKKMFDP KTSKVNKICD AVLNVLLSNP EYKKKYLQTI ITAYASQNP QNLSAALKLI SELENSEEKD SCVTYLCFLQ DVNVVYKSAL SLYDVSLALL VAQKSQMDPR EYLPFLQELQ D NEPLRRKF LIDDYLGNYE KALEHLSEID KDGNVSEEVI DYVESHDLYK HGLALYRYDS EKQNVIYNIY AKHLSSNQMY TD AAVAYEM LGKLKEAMGA YQSAKRWREA MSIAVQKFPE EVESVAEELI SSLTFEHRYV DAADIQLEYL DNVKEAVALY CKA YRYDIA SLVAIKAKKD ELLEEVVDPG LGEGFGIIAE LLADCKGQIN SQLRRLRELR AKKEENPYAF YGQETEQADD VSVA PSETS TQESFFTRYT GKTGGTAKTG ASRRTAKNKR REERKRARGK KGTIYEEEYL VQSVGRLIER LNQTKPDAVR VVEGL CRRN MREQAHQIQK NFVEVLDLLK ANVKEIYSIS EKDRERVNEN GEVYYIPEIP VPEIHDFPKS HIVDF UniProtKB: Elongator complex protein 1 |
-Macromolecule #2: Elongator complex protein 2
| Macromolecule | Name: Elongator complex protein 2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Strain: ATCC 204508 / S288c |
| Molecular weight | Theoretical: 89.51943 KDa |
| Sequence | String: MVECITPEAI FIGANKQTQV SDIHKVKKIV AFGAGKTIAL WDPIEPNNKG VYATLKGHEA EVTCVRFVPD SDFMVSASED HHVKIWKFT DYSHLQCIQT IQHYSKTIVA LSALPSLISV GCADGTISIW RQNIQNDEFG LAHEFTIKKG FFYPLCLSLS K VEEKKYLL ...String: MVECITPEAI FIGANKQTQV SDIHKVKKIV AFGAGKTIAL WDPIEPNNKG VYATLKGHEA EVTCVRFVPD SDFMVSASED HHVKIWKFT DYSHLQCIQT IQHYSKTIVA LSALPSLISV GCADGTISIW RQNIQNDEFG LAHEFTIKKG FFYPLCLSLS K VEEKKYLL AIGGTNVNVF IASFILSDSG IEKCRVVAEL EGHEDWVKSL AFRHQETPGD YLLCSGSQDR YIRLWRIRIN DL IDDSEED SKKLTLLSNK QYKFQIDDEL RVGINFEALI MGHDDWISSL QWHESRLQLL AATADTSLMV WEPDETSGIW VCS LRLGEM SSKGASTATG SSGGFWSCLW FTHERMDFFL TNGKTGSWRM WATKDNIICD QRLGISGATK DVTDIAWSPS GEYL LATSL DQTTRLFAPW IYDASGRKRE IATWHEFSRP QIHGYDMICV ETVTDTRFVS GGDEKILRSF DLPKGVAGML QKFVG IQFE EKSEMPDSAT VPVLGLSNKA GEDDANEDDE EEEGGNKETP DITDPLSLLE CPPMEDQLQR HLLWPEVEKL YGHGFE ITC LDISPDQKLI ASACRSNNVQ NAVIRIFSTE NWLEIKPALP FHSLTITRLK FSKDGKFLLS VCRDRKWALW ERNMEDN TF ELRFKNEKPH TRIIWDADWA PLEFGNVFVT ASRDKTVKVW RHQKEPADDY VLEASIKHTK AVTAISIHDS MIREKILI S VGLENGEIYL YSYTLGKFEL ITQLNEDITP ADKITRLRWS HLKRNGKLFL GVGSSDLSTR IYSLAYE UniProtKB: Elongator complex protein 2 |
-Macromolecule #3: Elongator complex protein 3
| Macromolecule | Name: Elongator complex protein 3 / type: protein_or_peptide / ID: 3 / Details: FeS cluster and 5DA / Number of copies: 1 / Enantiomer: LEVO / EC number: histone acetyltransferase |
|---|---|
| Source (natural) | Organism:  Strain: ATCC 204508 / S288c |
| Molecular weight | Theoretical: 63.755059 KDa |
| Sequence | String: MARHGKGPKT NKKKLAPEKE RFIQCCADIT LELTDSLTSG TTREINLNGL ITKYSKKYKL KQQPRLTDII NSIPDQYKKY LLPKLKAKP VRTASGIAVV AVMCKPHRCP HIAYTGNICV YCPGGPDSDF EYSTQSYTGY EPTSMRAIRA RYDPYEQARG R VEQLKQLG ...String: MARHGKGPKT NKKKLAPEKE RFIQCCADIT LELTDSLTSG TTREINLNGL ITKYSKKYKL KQQPRLTDII NSIPDQYKKY LLPKLKAKP VRTASGIAVV AVMCKPHRCP HIAYTGNICV YCPGGPDSDF EYSTQSYTGY EPTSMRAIRA RYDPYEQARG R VEQLKQLG HSIDKVEYVL MGGTFMSLPK EYREDFIVKL HNALSGFNGN DIDEAILYSQ QSLTKCVGIT IETRPDYCTQ TH LDDMLKY GCTRLEIGVQ SLYEDVARDT NRGHTVRSVC ETFAVSKDAG YKVVSHMMPD LPNVGMERDI EQFKEYFENP DFR TDGLKI YPTLVIRGTG LYELWKTGRY KSYSANALVD LVARILALVP PWTRIYRVQR DIPMPLVTSG VDNGNLRELA LARM KDLGT TCRDVRTREV GIQEVHHKVQ PDQVELIRRD YYANGGWETF LSYEDPKKDI LIGLLRLRKA SKKYTYRKEF TSQRT SIVR ELHVYGSVVP LHSRDPRKFQ HQGFGTLLME EAERIAKEEH GSEKISVISG VGVRNYYGKL GYELDGPYMS KRI UniProtKB: Elongator complex protein 3 |
-Macromolecule #4: IRON/SULFUR CLUSTER
| Macromolecule | Name: IRON/SULFUR CLUSTER / type: ligand / ID: 4 / Number of copies: 1 / Formula: SF4 |
|---|---|
| Molecular weight | Theoretical: 351.64 Da |
| Chemical component information |  ChemComp-FS1: |
-Macromolecule #5: 5'-DEOXYADENOSINE
| Macromolecule | Name: 5'-DEOXYADENOSINE / type: ligand / ID: 5 / Number of copies: 1 / Formula: 5AD |
|---|---|
| Molecular weight | Theoretical: 251.242 Da |
| Chemical component information |  ChemComp-5AD: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Details: Pelco EasyGlow glow discharger, 20 mA | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: 2.5 ul of sample, blotting parameters: wait time 15 s, blot force 5, blot time 5-8 s.. | ||||||||||||||||||
| Details | The sample was cross-linked with 0.01% glutaraldehyde, quenched and then plunged. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Details | Gatan Quantum energy filter and a K2 Summit direct detector |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Number real images: 4614 / Average electron dose: 43.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 105000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT | ||||||
| Output model |  PDB-6qk7: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)