+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4277 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM Structure INO80core Nucleosome complex | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | INO80 / Nucleosome / ATP dependent Chromatin Remodeller / DNA BINDING PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationHDACs deacetylate histones / HATs acetylate histones / RMTs methylate histone arginines / DASH complex / protein transport along microtubule to mitotic spindle pole body / mitotic sister chromatid biorientation / Metalloprotease DUBs / UCH proteinases / Ub-specific processing proteases / attachment of spindle microtubules to kinetochore ...HDACs deacetylate histones / HATs acetylate histones / RMTs methylate histone arginines / DASH complex / protein transport along microtubule to mitotic spindle pole body / mitotic sister chromatid biorientation / Metalloprotease DUBs / UCH proteinases / Ub-specific processing proteases / attachment of spindle microtubules to kinetochore / Swr1 complex / attachment of mitotic spindle microtubules to kinetochore / Ino80 complex / ATP-dependent activity, acting on DNA / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / Packaging Of Telomere Ends / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / telomere organization / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Interleukin-7 signaling / RNA Polymerase I Promoter Opening / Inhibition of DNA recombination at telomere / Assembly of the ORC complex at the origin of replication / Meiotic synapsis / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / innate immune response in mucosa / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / Negative Regulation of CDH1 Gene Transcription / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / Nonhomologous End-Joining (NHEJ) / RNA Polymerase I Promoter Escape / Transcriptional regulation by small RNAs / helicase activity / Formation of the beta-catenin:TCF transactivating complex / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / HDMs demethylate histones / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / G2/M DNA damage checkpoint / NoRC negatively regulates rRNA expression / kinetochore / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / DNA Damage/Telomere Stress Induced Senescence / Pre-NOTCH Transcription and Translation / Meiotic recombination / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Metalloprotease DUBs / Transcriptional regulation of granulopoiesis / RMTs methylate histone arginines / HCMV Early Events / mitotic spindle / structural constituent of chromatin / UCH proteinases / heterochromatin formation / nucleosome / antimicrobial humoral immune response mediated by antimicrobial peptide / nucleosome assembly / E3 ubiquitin ligases ubiquitinate target proteins / antibacterial humoral response / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / HATs acetylate histones / Factors involved in megakaryocyte development and platelet production / RUNX1 regulates transcription of genes involved in differentiation of HSCs / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / chromatin organization / Processing of DNA double-strand break ends / Senescence-Associated Secretory Phenotype (SASP) / gene expression / Oxidative Stress Induced Senescence / Estrogen-dependent gene expression / DNA helicase / chromosome, telomeric region / Ub-specific processing proteases / defense response to Gram-positive bacterium / chromatin remodeling / protein heterodimerization activity / Amyloid fiber formation / DNA repair / chromatin binding / enzyme binding / negative regulation of transcription by RNA polymerase II / ATP hydrolysis activity / protein-containing complex Similarity search - Function | ||||||||||||
| Biological species |  Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) / Chaetomium thermophilum var. thermophilum DSM 1495 (fungus) /  Homo sapiens (human) / synthetic construct (others) / Homo sapiens (human) / synthetic construct (others) /  Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus) Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus) | ||||||||||||
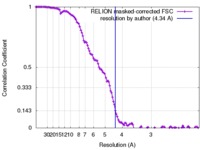
| Method | single particle reconstruction / cryo EM / Resolution: 4.34 Å | ||||||||||||
 Authors Authors | Eustermann S / Schall K | ||||||||||||
| Funding support |  Germany, 3 items Germany, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2018 Journal: Nature / Year: 2018Title: Structural basis for ATP-dependent chromatin remodelling by the INO80 complex. Authors: Sebastian Eustermann / Kevin Schall / Dirk Kostrewa / Kristina Lakomek / Mike Strauss / Manuela Moldt / Karl-Peter Hopfner /  Abstract: In the eukaryotic nucleus, DNA is packaged in the form of nucleosomes, each of which comprises about 147 base pairs of DNA wrapped around a histone protein octamer. The position and histone ...In the eukaryotic nucleus, DNA is packaged in the form of nucleosomes, each of which comprises about 147 base pairs of DNA wrapped around a histone protein octamer. The position and histone composition of nucleosomes is governed by ATP-dependent chromatin remodellers such as the 15-subunit INO80 complex . INO80 regulates gene expression, DNA repair and replication by sliding nucleosomes, the exchange of histone H2A.Z with H2A, and the positioning of + 1 and -1 nucleosomes at promoter DNA. The structures and mechanisms of these remodelling reactions are currently unknown. Here we report the cryo-electron microscopy structure of the evolutionarily conserved core of the INO80 complex from the fungus Chaetomium thermophilum bound to a nucleosome, at a global resolution of 4.3 Å and with major parts at 3.7 Å. The INO80 core cradles one entire gyre of the nucleosome through multivalent DNA and histone contacts. An Rvb1/Rvb2 AAA ATPase heterohexamer is an assembly scaffold for the complex and acts as a 'stator' for the motor and nucleosome-gripping subunits. The Swi2/Snf2 ATPase motor binds to nucleosomal DNA at superhelical location -6, unwraps approximately 15 base pairs, disrupts the H2A-DNA contacts and is poised to pump entry DNA into the nucleosome. Arp5 and Ies6 bind superhelical locations -2 and -3 to act as a counter grip for the motor, on the other side of the H2A-H2B dimer. The Arp5 insertion domain forms a grappler element that binds the nucleosome dyad, connects the Arp5 actin-fold and entry DNA over a distance of about 90 Å and packs against histone H2A-H2B near the 'acidic patch'. Our structure together with biochemical data suggests a unified mechanism for nucleosome sliding and histone editing by INO80. The motor is part of a macromolecular ratchet, persistently pumping entry DNA across the H2A-H2B dimer against the Arp5 grip until a large nucleosome translocation step occurs. The transient exposure of H2A-H2B by motor activity as well as differential recognition of H2A.Z and H2A may regulate histone exchange. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4277.map.gz emd_4277.map.gz | 88.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4277-v30.xml emd-4277-v30.xml emd-4277.xml emd-4277.xml | 34.7 KB 34.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4277_fsc.xml emd_4277_fsc.xml | 12.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_4277.png emd_4277.png | 63 KB | ||
| Filedesc metadata |  emd-4277.cif.gz emd-4277.cif.gz | 10.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4277 http://ftp.pdbj.org/pub/emdb/structures/EMD-4277 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4277 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4277 | HTTPS FTP |
-Related structure data
| Related structure data |  6fmlMC  4264C  4278C  4280C  6fhsC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4277.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4277.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : INO80core Nucleosome Complex
+Supramolecule #1: INO80core Nucleosome Complex
+Supramolecule #2: INO80core
+Supramolecule #3: Histone octamer
+Supramolecule #4: Synthetic deoxyribonucleic acid
+Macromolecule #1: RuvB-like helicase
+Macromolecule #2: RuvB-like helicase
+Macromolecule #3: Ino80
+Macromolecule #4: les2
+Macromolecule #5: Ies6
+Macromolecule #6: Actin related protein 5
+Macromolecule #9: Histone H3.2
+Macromolecule #10: Histone H4
+Macromolecule #11: Histone H2A type 1
+Macromolecule #12: Histone H2B type 1-C/E/F/G/I
+Macromolecule #7: Nucleosomal DNA Strand 1
+Macromolecule #8: Nucleosomal DNA Strand 2
+Macromolecule #13: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #14: ADENOSINE-5'-TRIPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 8 Details: 20 mM HEPES pH 8, 60 mM KCl, 0.5% glycerol, 0.25 mM CaCl2, 20 uM ZnCl2, 0.25 mM DTT, 0.05% Octyl-beta-glucoside |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 10 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 281 K / Instrument: LEICA EM GP |
| Details | Monodisperse sample: INO80core complex reconstituted with nucleosomal substrate was purified by gelfiltration. Addition of nucleotides or crosslinking was not required. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-40 / Number grids imaged: 1 / Number real images: 3992 / Average electron dose: 59.6 e/Å2 Details: Images were collected in movie mode with 4 frames per second and 10s total aquisition |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus min: 1.3 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)